Journal of the Taiwan Institute of Chemical Engineers ( IF 5.5 ) Pub Date : 2018-03-03 , DOI: 10.1016/j.jtice.2018.02.025 Zhike Wang , Yunyi Zhu , Haitian Chen , Haili Wu , Cunling Ye
|
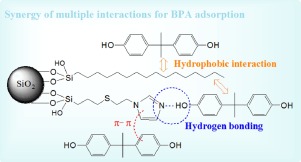
Based on the physicochemical properties of bisphenol A (BPA), chemical co-immobilizations of imidazole, phenyl and hexadecyl were done onto silica to obtain three functionalized silica adsorbents. Accordingly, the combinations of hexadecyl and imidazole, hexadecyl and phenyl, and phenyl and imidazole were named as Sil-(HDTMS-MPS)-VIm, Sil-(HDTMS-MPS)-S and Sil-MPS-(S-VIm), respectively. The composites were characterized by FTIR, TGA and SEM. The aim of this work was to explore how these typical functional groups affect the adsorption behavior of BPA. Experiments demonstrated that alkaline imidazole would play a key role for the BPA adsorption, and the synergy of imidazole and hexadecyl exhibited the highest adsorption affinities for BPA in the pH range of 4.0–9.0 due to the high Kow value of BPA. The maximum adsorption capacity of Sil-(HDTMS-MPS)-VIm for BPA obtained from Langmuir model was 89.6 mg/g at 293 K. Furthermore, the prepared three functionalized silicas retained their good adsorption capacities of BPA in high salt solutions, and the adsorption behaviors of BPA for these adsorbents were well described by the pseudo-second-order kinetic model and the Freundlich model. This work suggests that the synergy of hydrogen bond-forming alkaline functional group and hydrophobic long-chain alkyl group benefits BPA removal from aqueous solutions.
中文翻译:

三种功能化二氧化硅吸附剂的制备:咪唑,苯基和长链烷基共固定化对高盐水溶液中双酚A吸附的影响
基于双酚A(BPA)的理化性质,将咪唑,苯基和十六烷基化学共固定在二氧化硅上,得到三种官能化的二氧化硅吸附剂。因此,将十六烷基和咪唑,十六烷基和苯基以及苯基和咪唑的组合命名为Sil-(HDTMS-MPS)-VIm,Sil-(HDTMS-MPS)-S和Sil-MPS-(S-VIm),分别。通过FTIR,TGA和SEM对复合材料进行了表征。这项工作的目的是探讨这些典型的官能团如何影响BPA的吸附行为。实验表明,碱性咪唑对BPA的吸附起关键作用,并且由于高K ow值,咪唑和十六烷基的协同作用在pH 4.0-9.0范围内对BPA表现出最高的吸附亲和力。BPA的价值。从Langmuir模型获得的Sil-(HDTMS-MPS)-VIm对BPA的最大吸附容量在293 K下为89.6 mg / g。此外,制得的三种官能化二氧化硅在高盐溶液中保持了良好的BPA吸附能力。伪二级动力学模型和Freundlich模型很好地描述了BPA对这些吸附剂的吸附行为。这项工作表明形成氢键的碱性官能团和疏水性长链烷基的协同作用有益于从水溶液中去除BPA。











































 京公网安备 11010802027423号
京公网安备 11010802027423号