当前位置:
X-MOL 学术
›
Photochem. Photobiol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photophysical properties of benzanthrone derivatives: effect of substituent, solvent polarity and hydrogen bonding†
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1039/c7pp00392g Shivraj Shivraj 1, 2, 3, 4 , B. Siddlingeshwar 1, 2, 3, 4 , Elena M. Kirilova 5, 6, 7, 8 , Sergey V. Belyakov 8, 9, 10 , Darshan Devang Divakar 11, 12, 13, 14, 15 , Abdulaziz Abdullah Alkheraif 11, 12, 13, 14, 15
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1039/c7pp00392g Shivraj Shivraj 1, 2, 3, 4 , B. Siddlingeshwar 1, 2, 3, 4 , Elena M. Kirilova 5, 6, 7, 8 , Sergey V. Belyakov 8, 9, 10 , Darshan Devang Divakar 11, 12, 13, 14, 15 , Abdulaziz Abdullah Alkheraif 11, 12, 13, 14, 15
Affiliation
|
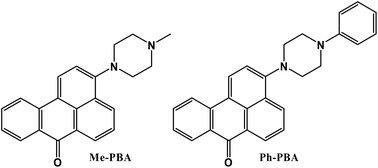
Benzanthrone derivatives are potential fluorescent probes for various chemical and biological environments. A mechanistic understanding of their photophysical properties is pivotal for designing an efficient fluorescence sensor based on the benzanthrone framework. In this study, we report on the effect of chemical substitution on the photophysical properties of two benzanthrone derivatives, namely, 3-(N′-methyl)-piperazino-7H-benzo[de]anthracen-7-one [Me-PBA] and 3-(N′-phenyl)-piperazino-7H-benzo[de]anthracen-7-one [Ph-PBA] in different solvents and solvent mixtures of varying polarities and proticities. Both benzanthrone derivatives show interesting solvent-dependent photophysical properties. Although both derivatives exhibit strong intramolecular charge transfer (ICT) characteristics in the excited state, the extent of the charge transfer is significantly influenced by the nature of the chemical substitution. Modulation of photophysical parameters as a function of solvent properties led us to propose that ICT is affected by solvent polarity and hydrogen bonding. From the viscosity effect, it is revealed that the weaker emission of Ph-PBA compared to Me-PBA in polar solvents is primarily due to the non-radiative torsional motion of the phenyl group in the former derivative. In protic solvents, intermolecular hydrogen bonding imparts strong non-radiative deactivation to both derivatives, thus rendering a weak fluorescence yield.
中文翻译:

苯并蒽醌衍生物的光物理性质:取代基,溶剂极性和氢键的影响†
苯并蒽醌衍生物是用于各种化学和生物环境的潜在荧光探针。机械理解其光物理性质对于基于苯并蒽酮框架设计高效的荧光传感器至关重要。在这项研究中,我们报告了化学取代对两种苯并蒽酮衍生物(3-(N'-甲基)-哌嗪子-7 H-苯并[de]蒽-7-一[Me-PBA]的光物理性质的影响]和3-(N'-苯基)-哌嗪子基7 H-苯并[蒽] -7-酮[Ph-PBA]在不同溶剂和极性和质子不同的溶剂混合物中。两种苯并蒽醌衍生物均显示出有趣的溶剂依赖性光物理性质。尽管两种衍生物在激发态下均显示出很强的分子内电荷转移(ICT)特性,但电荷转移的程度受化学取代性质的影响很大。光物理参数随溶剂性质的变化导致我们提出ICT受溶剂极性和氢键的影响。从粘度效应可以看出,在极性溶剂中,与Me-PBA相比,Ph-PBA的发射较弱,这主要是由于前一种衍生物中苯基的非辐射扭转运动引起的。在质子溶剂中
更新日期:2018-03-05
中文翻译:

苯并蒽醌衍生物的光物理性质:取代基,溶剂极性和氢键的影响†
苯并蒽醌衍生物是用于各种化学和生物环境的潜在荧光探针。机械理解其光物理性质对于基于苯并蒽酮框架设计高效的荧光传感器至关重要。在这项研究中,我们报告了化学取代对两种苯并蒽酮衍生物(3-(N'-甲基)-哌嗪子-7 H-苯并[de]蒽-7-一[Me-PBA]的光物理性质的影响]和3-(N'-苯基)-哌嗪子基7 H-苯并[蒽] -7-酮[Ph-PBA]在不同溶剂和极性和质子不同的溶剂混合物中。两种苯并蒽醌衍生物均显示出有趣的溶剂依赖性光物理性质。尽管两种衍生物在激发态下均显示出很强的分子内电荷转移(ICT)特性,但电荷转移的程度受化学取代性质的影响很大。光物理参数随溶剂性质的变化导致我们提出ICT受溶剂极性和氢键的影响。从粘度效应可以看出,在极性溶剂中,与Me-PBA相比,Ph-PBA的发射较弱,这主要是由于前一种衍生物中苯基的非辐射扭转运动引起的。在质子溶剂中











































 京公网安备 11010802027423号
京公网安备 11010802027423号