Journal of Power Sources ( IF 8.1 ) Pub Date : 2018-03-04 , DOI: 10.1016/j.jpowsour.2018.02.085 Kevin A. Hays , Rose E. Ruther , Alexander J. Kukay , Pengfei Cao , Tomonori Saito , David L. Wood , Jianlin Li
|
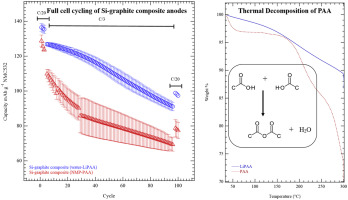
Lithium substituted polyacrylic acid (LiPAA) has previously been demonstrated as a superior binder over polyacrylic acid (PAA) for Si anodes, but from where does this enhanced performance arise? In this study, full cells are assembled with PAA and LiPAA based Si-graphite composite anodes that dried at temperatures from 100 °C to 200 °C. The performance of full cells containing PAA based Si-graphite anodes largely depend on the secondary drying temperature, as decomposition of the binder is correlated to increased electrode moisture and a rise in cell impedance. Full cells containing LiPAA based Si-graphite composite electrodes display better Coulombic efficiency than those with PAA, because of the electrochemical reduction of the PAA binder. This is identified by attenuated total reflectance Fourier transform infrared spectrometry and observed gassing during the electrochemical reaction. Coulombic losses from the PAA and Si SEI, along with depletion of the Si capacity in the anode results in progressive underutilization of the cathode and full cell capacity loss.
中文翻译:

是什么使锂取代的聚丙烯酸比硅-石墨复合阳极的聚丙烯酸更好的粘合剂?
锂取代的聚丙烯酸(LiPAA)先前已被证明是优于Si阳极的聚丙烯酸(PAA)的粘合剂,但是这种增强的性能从何而来呢?在这项研究中,将全电池与基于PAA和LiPAA的Si-石墨复合阳极组装在一起,并在100°C至200°C的温度下干燥。含有PAA基Si-石墨阳极的完整电池的性能在很大程度上取决于二次干燥温度,因为粘合剂的分解与电极湿度的增加和电池阻抗的升高相关。含LiPAA基Si-石墨复合电极的全电池比含PAA的全电池显示出更好的库仑效率,这是因为PAA粘合剂的电化学还原。这是通过衰减的全反射傅里叶变换红外光谱法和电化学反应期间观察到的放气来确定的。来自PAA和Si SEI的库仑损耗,以及阳极中Si容量的耗尽会导致阴极的逐步利用不足和整个电池容量的损失。











































 京公网安备 11010802027423号
京公网安备 11010802027423号