Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2018-03-02 , DOI: 10.1016/j.bmc.2018.03.002 Minhang Xin , Weiming Duan , Yifan Feng , Yuan-Yuan Hei , Hao Zhang , Ying Shen , Hong-Yi Zhao , Shuai Mao , San-Qi Zhang
|
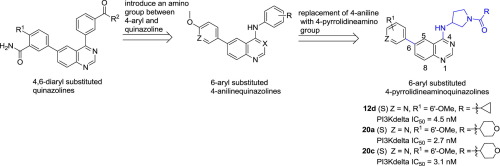
In this study, a novel series of 6-aryl substituted 4-pyrrolidineaminoquinazoline derivatives were designed and evaluated as potent PI3Kδ inhibitors. The preliminary SAR was established, and compounds 12d, 20a and 20c displayed leading potent PI3Kδ inhibition, with IC50 values of 4.5, 2.7 and 3.1 nM, respectively, that were comparable to idelalisib (IC50 = 2.7 nM). Moreover, these three compounds showed favorable PI3Kδ isoform selectivity over PI3Kα, PI3Kβ, and PI3Kγ, and showed distinct anti-proliferation profiles against four human B cell lines of Ramos, Raji, RPMI-8226 and SU-DHL-6. In addition, molecular docking simulation showed that several key hydrogen bonding interactions were formed for compounds 12d, 20a and 20c in the PI3Kδ pocket, which might explain their potent PI3Kδ inhibition. These results indicate the 6-aryl substituted 4-pyrrolidineaminoquinazolines were potent PI3Kδ inhibitors.
中文翻译:

新型6-芳基取代的4-吡咯烷氨基喹唑啉衍生物作为有效的磷酸肌醇3-激酶δ(PI3Kδ)抑制剂
在这项研究中,设计了一系列新型的6-芳基取代的4-吡咯烷氨基喹唑啉衍生物,并将其评估为有效的PI3Kδ抑制剂。建立了初步的SAR,化合物12d,20a和20c表现出领先的有效PI3Kδ抑制作用,IC 50值分别为4.5、2.7和3.1 nM,与艾屈拉西布相当(IC 50 = 2.7 nM)。此外,这三种化合物对PI3Kα,PI3Kβ和PI3Kγ的PI3Kδ同工型具有良好的选择性,并且对Ramos,Raji,RPMI-8226和SU-DHL-6的4种人类B细胞系均表现出独特的抗增殖特性。此外,分子对接模拟表明,化合物形成了几个关键的氢键相互作用PI3Kδ口袋中的12d,20a和20c可能解释了它们对PI3Kδ的有效抑制作用。这些结果表明6-芳基取代的4-吡咯烷氨基喹唑啉是有效的PI3Kδ抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号