当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Dynamics Study of Combustion Reactions in Supercritical Environment. Part 3: Boxed MD Study of CH3 + HO2 → CH3O + OH Reaction Kinetics
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acs.jpca.7b12233 Sergey V. Panteleev 1, 2 , Artëm E. Masunov 1, 3, 4, 5 , Subith S. Vasu 6
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acs.jpca.7b12233 Sergey V. Panteleev 1, 2 , Artëm E. Masunov 1, 3, 4, 5 , Subith S. Vasu 6
Affiliation
|
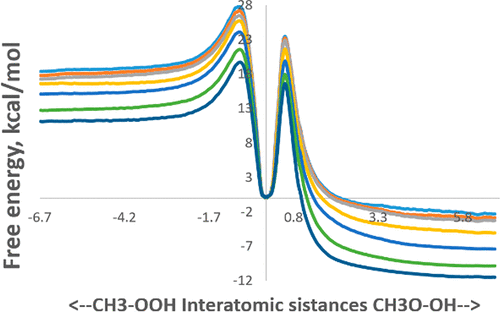
The kinetics of reaction CH3 + HO2 → CH3O + OH in supercritical carbon dioxide media at pressures from 0.3 to 1000 atm in the temperature range (600–1600) K was studied using boxed molecular dynamics simulations at QM/MM theory level with periodical boundary conditions. The mechanism of this process includes two consecutive steps: formation and decomposition of CH3OOH intermediate. We calculated the activation free energies and rate constants of each step, then used Bodenstein’s quasistationary concentrations approximation to estimate the rate constants of the reaction. On the basis of the temperature dependence of the rate constants, parameters in the extended Arrhenius equation were determined. We found that reaction rate of each step, as well as overall reaction, increases with increasing CO2 pressure in the system. The most effective zone for the process is T = 1000–1200 K, and the CO2 pressure is about 100 atm.
中文翻译:

超临界环境下燃烧反应的分子动力学研究。第3部分:CH 3 + HO 2 →CH 3 O + OH反应动力学的盒装MD研究
在QM / MM理论水平上使用盒装分子动力学模拟研究了在温度范围(600–1600)K在0.3至1000 atm的压力下,超临界二氧化碳介质中CH 3 + HO 2 →CH 3 O + OH的反应动力学。有周期性的边界条件。此过程的机理包括两个连续步骤:CH 3的形成和分解OOH中间体。我们计算了每个步骤的活化自由能和速率常数,然后使用Bodenstein的准静态浓度近似值来估计反应的速率常数。根据速率常数的温度依赖性,确定了扩展的Arrhenius方程中的参数。我们发现,随着系统中CO 2压力的增加,每个步骤的反应速率以及整个反应速率都会增加。该过程最有效的区域是T = 1000–1200 K,CO 2压力约为100 atm。
更新日期:2018-03-05
中文翻译:

超临界环境下燃烧反应的分子动力学研究。第3部分:CH 3 + HO 2 →CH 3 O + OH反应动力学的盒装MD研究
在QM / MM理论水平上使用盒装分子动力学模拟研究了在温度范围(600–1600)K在0.3至1000 atm的压力下,超临界二氧化碳介质中CH 3 + HO 2 →CH 3 O + OH的反应动力学。有周期性的边界条件。此过程的机理包括两个连续步骤:CH 3的形成和分解OOH中间体。我们计算了每个步骤的活化自由能和速率常数,然后使用Bodenstein的准静态浓度近似值来估计反应的速率常数。根据速率常数的温度依赖性,确定了扩展的Arrhenius方程中的参数。我们发现,随着系统中CO 2压力的增加,每个步骤的反应速率以及整个反应速率都会增加。该过程最有效的区域是T = 1000–1200 K,CO 2压力约为100 atm。











































 京公网安备 11010802027423号
京公网安备 11010802027423号