当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Investigation of the Gas-Phase SN2 Reactions of Anionic and Neutral Nucleophiles with Chloramines
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acs.jpca.7b11780 Jieqing Liu 1 , Meng Dong 1 , Shuo Zhang 1 , Yong Dong Liu 1 , Rugang Zhong 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acs.jpca.7b11780 Jieqing Liu 1 , Meng Dong 1 , Shuo Zhang 1 , Yong Dong Liu 1 , Rugang Zhong 1
Affiliation
|
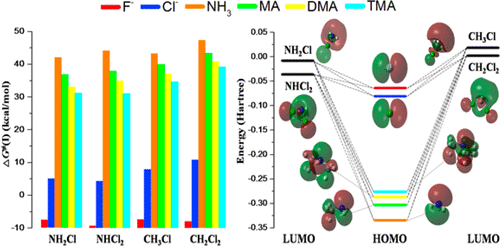
The SN2 reactions at nitrogen center (SN[email protected]) play a significant role in organic synthesis, carcinogenesis, and the formation of some environmentally toxic compounds. However, the SN[email protected] reactions specifically for neutral compounds as nucleophiles are less known. In this work, reactions of dimethylamine (DMA) and F– with NH2Cl were investigated as model reactions to validate an accurate functional from 24 DFT functionals by comparing with the CCSD(T) reference data. M06-2X functional was found to perform best and applied to systematically explore the trends in reactivity for halides (F– and Cl–) and simple amines toward the substrates NH2Cl and NHCl2 (SN[email protected]) as well as CH3Cl and CH2Cl2 (SN[email protected]). The computational results show that the backside inversion channel dominates most the SN[email protected] reactions except for the case of F– + NHCl2, which reacts preferentially via proton transfer. The overall activation free energies (ΔG‡) of the inversion channel for the SN2 reactions of F– and Cl– with chloramines are negative, whereas those for amines as nucleophiles are around 30–44 kcal/mol. The SN[email protected] reactions for all the nucleophiles investigated here are faster than the corresponding SN[email protected] Moreover, amines react faster when they have a higher extent of methyl substitution. Additionally, the energy gap between the HOMO of nucleophile and LUMO of substrate generally correlates well with ΔG‡ of the corresponding SN2 reactions, which is consistent with previous results.
中文翻译:

阴离子和中性亲核试剂与氯胺气相S N 2反应的理论研究
氮中心的S N 2反应(S N [电子邮件保护])在有机合成,致癌作用以及某些对环境有毒的化合物的形成中起着重要作用。但是,S Ñ [电子邮件保护]反应专门为中性的化合物作为亲核试剂是以下公知的。在这项工作中,以二甲胺(DMA)和F –与NH 2 Cl的反应为模型反应进行了研究,以通过与CCSD(T)参考数据进行比较,从24种DFT官能团中验证准确的官能团。发现M06-2X功能最佳,可用于系统探索卤化物(F –和Cl –)和朝向基板NH简单胺2 Cl和NHCl 2(S Ñ [电子邮件保护]),以及CH 3 Cl和CH 2氯2(S Ñ [电子邮件保护])。计算结果表明,除F – + NHCl 2优先通过质子转移反应外,背面反型通道主导了大多数的S N [电子邮件保护]反应。F –和Cl –的S N 2反应的反型通道的总活化自由能(ΔG ‡)。含氯胺的胺为阴性,而作为亲核试剂的胺为约30–44 kcal / mol。此处研究的所有亲核试剂的S N [电子邮件保护]反应比相应的S N [电子邮件保护]反应更快。此外,当胺具有较高的甲基取代度时,它们的反应也会更快。另外,亲核试剂的HOMO和底物的LUMO之间的能隙通常与相应的S N 2反应的ΔG ‡很好地相关,这与先前的结果一致。
更新日期:2018-03-02
中文翻译:

阴离子和中性亲核试剂与氯胺气相S N 2反应的理论研究
氮中心的S N 2反应(S N [电子邮件保护])在有机合成,致癌作用以及某些对环境有毒的化合物的形成中起着重要作用。但是,S Ñ [电子邮件保护]反应专门为中性的化合物作为亲核试剂是以下公知的。在这项工作中,以二甲胺(DMA)和F –与NH 2 Cl的反应为模型反应进行了研究,以通过与CCSD(T)参考数据进行比较,从24种DFT官能团中验证准确的官能团。发现M06-2X功能最佳,可用于系统探索卤化物(F –和Cl –)和朝向基板NH简单胺2 Cl和NHCl 2(S Ñ [电子邮件保护]),以及CH 3 Cl和CH 2氯2(S Ñ [电子邮件保护])。计算结果表明,除F – + NHCl 2优先通过质子转移反应外,背面反型通道主导了大多数的S N [电子邮件保护]反应。F –和Cl –的S N 2反应的反型通道的总活化自由能(ΔG ‡)。含氯胺的胺为阴性,而作为亲核试剂的胺为约30–44 kcal / mol。此处研究的所有亲核试剂的S N [电子邮件保护]反应比相应的S N [电子邮件保护]反应更快。此外,当胺具有较高的甲基取代度时,它们的反应也会更快。另外,亲核试剂的HOMO和底物的LUMO之间的能隙通常与相应的S N 2反应的ΔG ‡很好地相关,这与先前的结果一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号