当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pH Effects on the Selectivity of the Electrocatalytic CO2 Reduction on Graphene-Embedded Fe–N–C Motifs: Bridging Concepts between Molecular Homogeneous and Solid-State Heterogeneous Catalysis
ACS Energy Letters ( IF 19.3 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acsenergylett.8b00273 Ana Sofia Varela 1, 2 , Matthias Kroschel 2 , Nathaniel D. Leonard 2 , Wen Ju 2 , Julian Steinberg 2 , Alexander Bagger 3 , Jan Rossmeisl 3 , Peter Strasser 2
ACS Energy Letters ( IF 19.3 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acsenergylett.8b00273 Ana Sofia Varela 1, 2 , Matthias Kroschel 2 , Nathaniel D. Leonard 2 , Wen Ju 2 , Julian Steinberg 2 , Alexander Bagger 3 , Jan Rossmeisl 3 , Peter Strasser 2
Affiliation
|
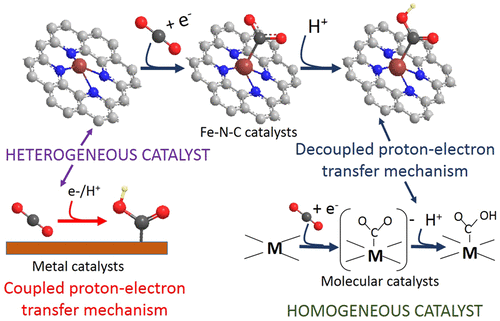
The electrochemical CO2 reduction reaction (CO2RR) is a promising route for converting CO2 and excess renewable energy into valuable chemicals and synthetic fuels. Recently, carbon-based solid materials containing dopant-levels of transition metal and nitrogen (M–N–C) have emerged as a cost-effective, energy-efficient catalyst for the direct coreduction of CO2 and H2O to CO. Fe–N–C catalysts are particularly interesting as they can reduce CO further to hydrocarbons. Despite these promising reports, the influence of the reaction conditions on the catalytic performance of Fe–N–C catalysts has not been addressed. Here, we study the role of the electrolyte on the CO2RR selectivity. Unlike hydrogen or methane generation, catalytic CO production is independent of the electrolyte pH on the normal hydrogen electrode potential scale, suggesting a decoupled elementary proton–electron transfer mechanism for CO formation. The similarity between this heterogeneous charge-transfer reaction mechanism and that of molecular metal–nitrogen porphyrin-type macrocyclic complexes strongly suggests that the carbon-embedded FeNx motifs of the solid-state electrocatalyst act as the primary catalytically active center and illustrates yet another example of unifying concepts between molecular and solid-state catalysis.
中文翻译:

pH值对嵌入石墨烯的Fe–N–C基序上电催化CO 2还原选择性的影响:分子均相和固态多相催化之间的桥接概念
电化学的CO 2还原反应(CO 2 RR)是将CO 2和过量的可再生能源转化为有价值的化学品和合成燃料的有前途的途径。最近,含有掺杂剂水平的过渡金属和氮(MC–N)的碳基固体材料已经成为一种经济高效,节能的催化剂,可将CO 2和H 2 O直接共轭成CO。 –N–C催化剂特别有趣,因为它们可以将CO进一步还原为碳氢化合物。尽管有这些令人鼓舞的报道,但尚未解决反应条件对Fe–N–C催化剂催化性能的影响。在这里,我们研究了电解质对CO 2的作用RR选择性。与产生氢气或甲烷不同,在正常的氢电极电势规模上,催化性CO的产生与电解质的pH无关,这表明用于CO形成的元素质子-电子传递机理是解耦的。这种异质电荷转移反应机理与分子金属-氮卟啉型大环配合物之间的相似性强烈表明,固态电催化剂的碳嵌入FeN x基序充当了主要的催化活性中心,并举例说明了另一个例子。分子催化和固态催化的统一概念
更新日期:2018-03-05
中文翻译:

pH值对嵌入石墨烯的Fe–N–C基序上电催化CO 2还原选择性的影响:分子均相和固态多相催化之间的桥接概念
电化学的CO 2还原反应(CO 2 RR)是将CO 2和过量的可再生能源转化为有价值的化学品和合成燃料的有前途的途径。最近,含有掺杂剂水平的过渡金属和氮(MC–N)的碳基固体材料已经成为一种经济高效,节能的催化剂,可将CO 2和H 2 O直接共轭成CO。 –N–C催化剂特别有趣,因为它们可以将CO进一步还原为碳氢化合物。尽管有这些令人鼓舞的报道,但尚未解决反应条件对Fe–N–C催化剂催化性能的影响。在这里,我们研究了电解质对CO 2的作用RR选择性。与产生氢气或甲烷不同,在正常的氢电极电势规模上,催化性CO的产生与电解质的pH无关,这表明用于CO形成的元素质子-电子传递机理是解耦的。这种异质电荷转移反应机理与分子金属-氮卟啉型大环配合物之间的相似性强烈表明,固态电催化剂的碳嵌入FeN x基序充当了主要的催化活性中心,并举例说明了另一个例子。分子催化和固态催化的统一概念











































 京公网安备 11010802027423号
京公网安备 11010802027423号