Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intratumoral Injection of Low-Energy Photon-Emitting Gold Nanoparticles: A Microdosimetric Monte Carlo-Based Model
ACS Nano ( IF 15.8 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acsnano.7b08242 Myriam Laprise-Pelletier 1, 2 , Yunzhi Ma 3 , Jean Lagueux 1 , Marie-France Côté 1 , Luc Beaulieu 3, 4 , Marc-André Fortin 1, 2
ACS Nano ( IF 15.8 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acsnano.7b08242 Myriam Laprise-Pelletier 1, 2 , Yunzhi Ma 3 , Jean Lagueux 1 , Marie-France Côté 1 , Luc Beaulieu 3, 4 , Marc-André Fortin 1, 2
Affiliation
|
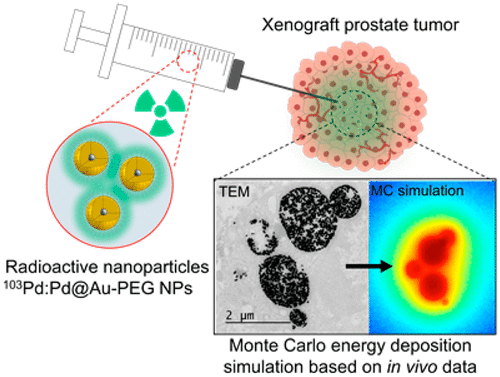
Gold nanoparticles (Au NPs) distributed in the vicinity of low-dose rate (LDR) brachytherapy seeds could multiply their efficacy thanks to the secondary emissions induced by the photoelectric effect. Injections of radioactive LDR gold nanoparticles (LDR Au NPs), instead of conventional millimeter-size radioactive seeds surrounded by Au NPs, could further enhance the dose by distributing the radioactivity more precisely and homogeneously in tumors. However, the potential of LDR Au NPs as an emerging strategy to treat cancer is strongly dependent on the macroscopic diffusion of the NPs in tumors, as well as on their microscopic internalization within the cells. Understanding the relationship between interstitial and intracellular distribution of NPs, and the outcomes of dose deposition in the cancer tissue is essential for considering future applications of radioactive Au NPs in oncology. Here, LDR Au NPs (103Pd:[email protected] NPs) were injected in prostate cancer tumors. The particles were visualized at time-points by computed tomography imaging (in vivo), transmission electron microscopy (ex vivo), and optical microscopy (ex vivo). These data were used in a Monte Carlo-based dosimetric model to reveal the dose deposition produced by LDR Au NPs both at tumoral and cellular scales. 103Pd:[email protected] NPs injected in tumors produce a strong dose enhancement at the intracellular level. However, energy deposition is mainly confined around vesicles filled with NPs, and not necessarily close to the nuclei. This suggests that indirect damage caused by the production of reactive oxygen species might be the leading therapeutic mechanism of tumor growth control, over direct damage to the DNA.
中文翻译:

低能量光子发射金纳米粒子的瘤内注射:基于微剂量法的蒙特卡洛模型
由于光电效应引起的二次发射,分布在低剂量率(LDR)近距离放射治疗种子附近的金纳米颗粒(Au NPs)可以提高其功效。注射放射性LDR金纳米粒子(LDR Au NPs)代替被Au NPs包围的常规毫米大小的放射性种子,可以通过在肿瘤中更精确,更均匀地分布放射性来进一步提高剂量。然而,LDR Au NPs作为治疗癌症的新兴策略的潜力在很大程度上取决于NPs在肿瘤中的宏观扩散,以及它们在细胞内的微观内在化。了解NP的间隙和细胞内分布之间的关系,癌症组织中剂量沉积的结果对于考虑放射性Au NP在肿瘤学中的未来应用至关重要。在这里,LDR Au NPs(在前列腺癌肿瘤中注射了103 Pd:[受电子邮件保护的NPs]。通过计算机断层摄影成像(体内),透射电子显微镜(离体)和光学显微镜(离体)在时间点观察颗粒。这些数据用于基于蒙特卡洛的剂量学模型中,以揭示LDR Au NP在肿瘤和细胞尺度上产生的剂量沉积。103Pd:[电子邮件保护的]注射到肿瘤中的NP在细胞内水平上产生了强烈的剂量增加。但是,能量沉积主要局限于充满NP的囊泡,并且不一定靠近细胞核。这表明,与对DNA的直接破坏相比,由活性氧物质的产生引起的间接破坏可能是肿瘤生长控制的主要治疗机制。
更新日期:2018-03-02
中文翻译:

低能量光子发射金纳米粒子的瘤内注射:基于微剂量法的蒙特卡洛模型
由于光电效应引起的二次发射,分布在低剂量率(LDR)近距离放射治疗种子附近的金纳米颗粒(Au NPs)可以提高其功效。注射放射性LDR金纳米粒子(LDR Au NPs)代替被Au NPs包围的常规毫米大小的放射性种子,可以通过在肿瘤中更精确,更均匀地分布放射性来进一步提高剂量。然而,LDR Au NPs作为治疗癌症的新兴策略的潜力在很大程度上取决于NPs在肿瘤中的宏观扩散,以及它们在细胞内的微观内在化。了解NP的间隙和细胞内分布之间的关系,癌症组织中剂量沉积的结果对于考虑放射性Au NP在肿瘤学中的未来应用至关重要。在这里,LDR Au NPs(在前列腺癌肿瘤中注射了103 Pd:[受电子邮件保护的NPs]。通过计算机断层摄影成像(体内),透射电子显微镜(离体)和光学显微镜(离体)在时间点观察颗粒。这些数据用于基于蒙特卡洛的剂量学模型中,以揭示LDR Au NP在肿瘤和细胞尺度上产生的剂量沉积。103Pd:[电子邮件保护的]注射到肿瘤中的NP在细胞内水平上产生了强烈的剂量增加。但是,能量沉积主要局限于充满NP的囊泡,并且不一定靠近细胞核。这表明,与对DNA的直接破坏相比,由活性氧物质的产生引起的间接破坏可能是肿瘤生长控制的主要治疗机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号