当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transition-Metal-Free C-Vinylation of Ketones with Acetylenes: A Quantum-Chemical Rationalization of Similarities and Differences in Catalysis by Superbases MOH/DMSO and tBuOM/DMSO (M = Na, K)
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acs.joc.8b00071 Vladimir B. Orel 1, 2 , Nadezhda M. Vitkovskaya 1 , Vladimir B. Kobychev 1 , Boris A. Trofimov 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acs.joc.8b00071 Vladimir B. Orel 1, 2 , Nadezhda M. Vitkovskaya 1 , Vladimir B. Kobychev 1 , Boris A. Trofimov 2
Affiliation
|
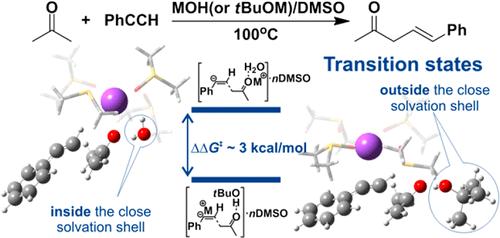
Transition-metal-free C-vinylation of acetone with phenylacetylene catalyzed by superbases MOH/DMSO and tBuOM/DMSO (M = Na, K) has been theoretically evaluated in the B3LYP/6-311++G**//B3LYP/6-31+G* approach to rationalize similarities and differences in activity of the above catalytic systems. The close solvate surroundings of sodium and potassium tert-butoxides have been studied. Formation of tBuOM·nDMSO complexes and their structure and thermodynamic stability are discussed in comparison with similar complexes of alkali-metal hydroxides MOH·nDMSO. Activation barriers of the title reaction in the presence of tBuOM·nDMSO complexes are found to be less than those with MOH·nDMSO complexes participating.
中文翻译:

酮与乙炔的无过渡金属C-文尼化:超级碱MOH / DMSO和t BuOM / DMSO催化相似性和差异的量子化学合理化(M = Na,K)
在B3LYP / 6-311 ++ G ** // B3LYP /中,对超碱MOH / DMSO和t BuOM / DMSO(M = Na,K)催化的丙酮与苯乙炔的无过渡金属C乙烯基化进行了评估。 6-31 + G *方法可合理化上述催化系统活性的相似之处和不同之处。已经研究了叔丁醇钠和叔丁醇钾的紧密溶剂化物环境。与碱金属氢氧化物MOH· n DMSO的类似配合物相比,讨论了t BuOM· n DMSO配合物的形成,结构和热力学稳定性。t BuOM· n存在下标题反应的激活障碍发现DMSO配合物少于MOH· n DMSO配合物。
更新日期:2018-03-05
中文翻译:

酮与乙炔的无过渡金属C-文尼化:超级碱MOH / DMSO和t BuOM / DMSO催化相似性和差异的量子化学合理化(M = Na,K)
在B3LYP / 6-311 ++ G ** // B3LYP /中,对超碱MOH / DMSO和t BuOM / DMSO(M = Na,K)催化的丙酮与苯乙炔的无过渡金属C乙烯基化进行了评估。 6-31 + G *方法可合理化上述催化系统活性的相似之处和不同之处。已经研究了叔丁醇钠和叔丁醇钾的紧密溶剂化物环境。与碱金属氢氧化物MOH· n DMSO的类似配合物相比,讨论了t BuOM· n DMSO配合物的形成,结构和热力学稳定性。t BuOM· n存在下标题反应的激活障碍发现DMSO配合物少于MOH· n DMSO配合物。









































 京公网安备 11010802027423号
京公网安备 11010802027423号