当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unlocking the Potential of Phenacyl Protecting Groups: CO2-Based Formation and Photocatalytic Release of Caged Amines
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acs.joc.8b00096 Elisabeth Speckmeier 1 , Michael Klimkait 1 , Kirsten Zeitler 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acs.joc.8b00096 Elisabeth Speckmeier 1 , Michael Klimkait 1 , Kirsten Zeitler 1
Affiliation
|
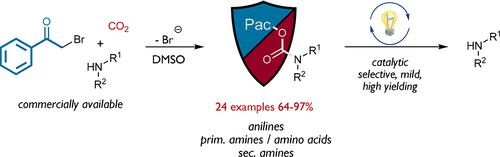
Orthogonal protection and deprotection of amines remain important tools in synthetic design as well as in chemical biology and material research applications. A robust, highly efficient, and sustainable method for the formation of phenacyl-based carbamate esters was developed using CO2 for the in situ preparation of the intermediate carbamates. Our mild and broadly applicable protocol allows for the formation of phenacyl urethanes of anilines, primary amines, including amino acids, and secondary amines in high to excellent yields. Moreover, we demonstrate the utility by a mild and convenient photocatalytic deprotection protocol using visible light. A key feature of the [Ru(bpy)3](PF6)2-catalyzed method is the use of ascorbic acid as reductive quencher in a neutral, buffered, two-phase acetonitrile/water mixture, granting fast and highly selective deprotection for all presented examples.
中文翻译:

释放苯基保护基的潜力:基于CO 2的形成和笼状胺的光催化释放
胺的正交保护和脱保护仍然是合成设计以及化学生物学和材料研究应用中的重要工具。开发了一种强大,高效且可持续的方法,用于形成基于苯甲酰基氨基甲酸酯的原位制备中间氨基甲酸酯的过程中使用了CO 2。我们温和且广泛适用的方案允许以高到极好的收率形成苯胺,伯胺(包括氨基酸)和仲胺的苯甲酰氨基甲酸酯。此外,我们通过使用可见光的温和便捷的光催化脱保护方案展示了该实用程序。[Ru(bpy)3 ](PF 6)2的关键特征-催化的方法是在中性,缓冲的两相乙腈/水混合物中使用抗坏血酸作为还原性淬灭剂,从而为所有给出的实施例提供快速且高度选择性的脱保护。
更新日期:2018-03-05
中文翻译:

释放苯基保护基的潜力:基于CO 2的形成和笼状胺的光催化释放
胺的正交保护和脱保护仍然是合成设计以及化学生物学和材料研究应用中的重要工具。开发了一种强大,高效且可持续的方法,用于形成基于苯甲酰基氨基甲酸酯的原位制备中间氨基甲酸酯的过程中使用了CO 2。我们温和且广泛适用的方案允许以高到极好的收率形成苯胺,伯胺(包括氨基酸)和仲胺的苯甲酰氨基甲酸酯。此外,我们通过使用可见光的温和便捷的光催化脱保护方案展示了该实用程序。[Ru(bpy)3 ](PF 6)2的关键特征-催化的方法是在中性,缓冲的两相乙腈/水混合物中使用抗坏血酸作为还原性淬灭剂,从而为所有给出的实施例提供快速且高度选择性的脱保护。











































 京公网安备 11010802027423号
京公网安备 11010802027423号