当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst-Directed Guidance of Sulfur-Substituted Enediolates to Stereoselective Carbon-Carbon Bond Formation with Aldehydes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-04 , DOI: 10.1021/jacs.7b12949 Daisuke Uraguchi 1 , Kohei Yamada 1 , Makoto Sato 2 , Takashi Ooi 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-04 , DOI: 10.1021/jacs.7b12949 Daisuke Uraguchi 1 , Kohei Yamada 1 , Makoto Sato 2 , Takashi Ooi 1, 2
Affiliation
|
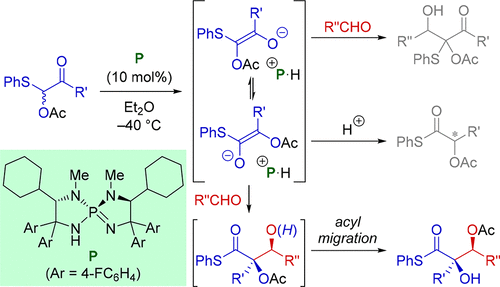
A highly chemo-, regio-, and stereoselective glycolate aldol reaction of sulfur-substituted enediolates with aldehydes was developed by employing a l-cyclohexylglycine-derived chiral iminophosphorane as a catalyst. The key for establishing this protocol is the distinct ability of the iminophosphorane catalyst to precisely direct the equilibrium mixture of the enediolates toward the intermolecular carbon-carbon bond formation with simultaneous yet rigorous control of relative and absolute stereochemistry. The critical importance of the cyclohexyl substituents on the catalyst backbone in dictating the reaction pathway and the stereochemical outcome was elucidated through an extensive quantum analysis by density functional theory calculations.
中文翻译:

硫取代烯二醇的催化剂导向与醛的立体选择性碳-碳键形成
通过使用 L-环己基甘氨酸衍生的手性亚氨基正膦作为催化剂,开发了硫取代的烯二醇与醛的高度化学、区域和立体选择性乙醇酸醛醇反应。建立该协议的关键是亚氨基膦催化剂的独特能力,可以精确地将烯二醇的平衡混合物导向分子间碳-碳键的形成,同时严格控制相对和绝对立体化学。通过密度泛函理论计算的广泛量子分析阐明了催化剂骨架上的环己基取代基在决定反应途径和立体化学结果方面的关键重要性。
更新日期:2018-03-04
中文翻译:

硫取代烯二醇的催化剂导向与醛的立体选择性碳-碳键形成
通过使用 L-环己基甘氨酸衍生的手性亚氨基正膦作为催化剂,开发了硫取代的烯二醇与醛的高度化学、区域和立体选择性乙醇酸醛醇反应。建立该协议的关键是亚氨基膦催化剂的独特能力,可以精确地将烯二醇的平衡混合物导向分子间碳-碳键的形成,同时严格控制相对和绝对立体化学。通过密度泛函理论计算的广泛量子分析阐明了催化剂骨架上的环己基取代基在决定反应途径和立体化学结果方面的关键重要性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号