当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lewis Acid Activation of the Ferrous Heme–NO Fragment Towards the N–N Coupling Reaction with NO to Generate N2O
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-04 , DOI: 10.1021/jacs.7b13681 Erwin G. Abucayon 1 , Rahul L. Khade 2 , Douglas R. Powell 1 , Yong Zhang 2 , George B. Richter-Addo 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-04 , DOI: 10.1021/jacs.7b13681 Erwin G. Abucayon 1 , Rahul L. Khade 2 , Douglas R. Powell 1 , Yong Zhang 2 , George B. Richter-Addo 1
Affiliation
|
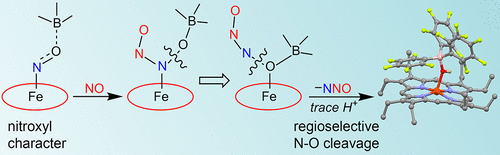
Bacterial NO reductase (bacNOR) enzymes utilize a heme/non-heme active site to couple two NO molecules to N2O. We show that BF3 coordination to the nitrosyl O-atom in (OEP)Fe(NO) activates it toward N-N bond formation with NO to generate N2O. 15N-isotopic labeling reveals a reversible nitrosyl exchange reaction and follow-up N-O bond cleavage in the N2O formation step. Other Lewis acids (B(C6F5)3 and K+) also promote the NO coupling reaction with (OEP)Fe(NO). These results, complemented by DFT calculations, provide experimental support for the cis: b3 pathway in bacNOR.
中文翻译:

亚铁血红素-NO 片段的路易斯酸活化朝向与 NO 的 N-N 偶联反应生成 N2O
细菌 NO 还原酶 (bacNOR) 酶利用血红素/非血红素活性位点将两个 NO 分子与 N2O 偶联。我们表明,BF3 与 (OEP)Fe(NO) 中亚硝酰基 O 原子的配位将其激活,使其与 NO 形成 NN 键以生成 N2O。15N 同位素标记揭示了在 N2O 形成步骤中可逆的亚硝酰基交换反应和后续的 NO 键断裂。其他路易斯酸(B(C6F5)3 和 K+)也促进 NO 与 (OEP)Fe(NO) 的偶联反应。这些结果与 DFT 计算相辅相成,为 bacNOR 中的 cis: b3 通路提供了实验支持。
更新日期:2018-03-04
中文翻译:

亚铁血红素-NO 片段的路易斯酸活化朝向与 NO 的 N-N 偶联反应生成 N2O
细菌 NO 还原酶 (bacNOR) 酶利用血红素/非血红素活性位点将两个 NO 分子与 N2O 偶联。我们表明,BF3 与 (OEP)Fe(NO) 中亚硝酰基 O 原子的配位将其激活,使其与 NO 形成 NN 键以生成 N2O。15N 同位素标记揭示了在 N2O 形成步骤中可逆的亚硝酰基交换反应和后续的 NO 键断裂。其他路易斯酸(B(C6F5)3 和 K+)也促进 NO 与 (OEP)Fe(NO) 的偶联反应。这些结果与 DFT 计算相辅相成,为 bacNOR 中的 cis: b3 通路提供了实验支持。











































 京公网安备 11010802027423号
京公网安备 11010802027423号