当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solution Thermodynamics of Benzotriazole in Different Pure Solvents
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acs.jced.7b01085 Yanan Luan 1, 2 , Jing Li 1 , Musika Kaliwanda 1 , Na Wang 1 , Kui Chen 1 , Xin Li 1 , Weiyi Su 3 , Hongxun Hao 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acs.jced.7b01085 Yanan Luan 1, 2 , Jing Li 1 , Musika Kaliwanda 1 , Na Wang 1 , Kui Chen 1 , Xin Li 1 , Weiyi Su 3 , Hongxun Hao 1, 2
Affiliation
|
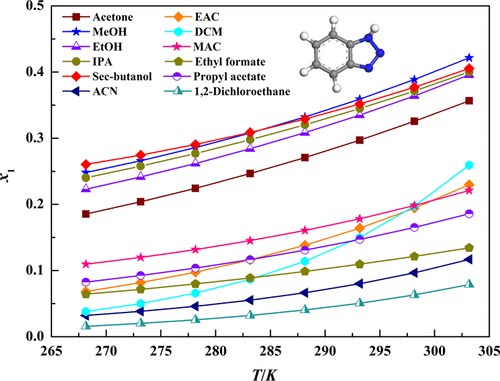
Solubility data of benzotriazole (BTA) in 14 solvents were measured by a static gravimetric method from 268.15 to 303.15 K. The results illustrated that the solubility of BTA in N,N-dimethylacetamide is the highest whereas that in 1,2-dichloroethane is the lowest in all the tested solvents. And the values of solubility increase with increasing of temperature. Additionally, the modified Apelblat equation, the λh equation, the NRTL model, and the Wilson model were employed to correlate the experimental solubility data. It was found that all the selected thermodynamic models could give satisfactory correlation results. Furthermore, the thermodynamic properties of BTA during the dissolving process, including the enthalpy, the Gibbs energy, and the entropy, were also calculated and analyzed.
中文翻译:

苯并三唑在不同纯溶剂中的溶液热力学
采用静态重量法在268.15至303.15 K之间测量了苯并三唑(BTA)在14种溶剂中的溶解度数据。结果表明,BTA在N,N-二甲基乙酰胺中的溶解度最高,而在1,2-二氯乙烷中的溶解度最高。在所有测试的溶剂中含量最低。并且溶解度的值随着温度的升高而增加。此外,修改后的Apelblat方程λh方程,NRTL模型和Wilson模型用于关联实验溶解度数据。发现所有选择的热力学模型都可以给出令人满意的相关结果。此外,还计算和分析了BTA在溶解过程中的热力学性质,包括焓,吉布斯能和熵。
更新日期:2018-03-06
中文翻译:

苯并三唑在不同纯溶剂中的溶液热力学
采用静态重量法在268.15至303.15 K之间测量了苯并三唑(BTA)在14种溶剂中的溶解度数据。结果表明,BTA在N,N-二甲基乙酰胺中的溶解度最高,而在1,2-二氯乙烷中的溶解度最高。在所有测试的溶剂中含量最低。并且溶解度的值随着温度的升高而增加。此外,修改后的Apelblat方程λh方程,NRTL模型和Wilson模型用于关联实验溶解度数据。发现所有选择的热力学模型都可以给出令人满意的相关结果。此外,还计算和分析了BTA在溶解过程中的热力学性质,包括焓,吉布斯能和熵。











































 京公网安备 11010802027423号
京公网安备 11010802027423号