当前位置:
X-MOL 学术
›
Environ. Sci. Technol. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Quantitative Model for the Coupled Kinetics of Arsenic Adsorption/Desorption and Oxidation on Manganese Oxides
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acs.estlett.8b00058 Xionghan Feng 1 , Pei Wang 2 , Zhenqing Shi 2 , Kideok D. Kwon 3 , Huaiyan Zhao 1 , Hui Yin 1 , Zhang Lin 2 , Mengqiang Zhu 4 , Xinran Liang 1 , Fan Liu 1 , Donald L. Sparks 5
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acs.estlett.8b00058 Xionghan Feng 1 , Pei Wang 2 , Zhenqing Shi 2 , Kideok D. Kwon 3 , Huaiyan Zhao 1 , Hui Yin 1 , Zhang Lin 2 , Mengqiang Zhu 4 , Xinran Liang 1 , Fan Liu 1 , Donald L. Sparks 5
Affiliation
|
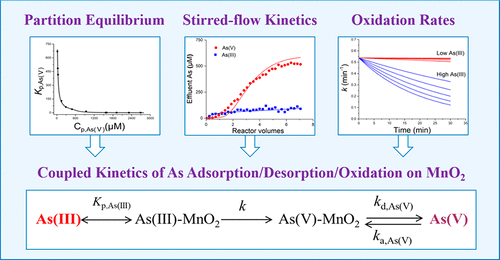
The ability to predict coupled kinetic reactions of arsenic (As) that occur at the manganese oxide–aqueous solution interface is crucial for management and remediation of As-contaminated sites worldwide. A quantitative understanding of the coupling between As oxidation and adsorption/desorption kinetics will enable us to more accurately predict the kinetic behavior of As. In this study, we developed a novel quantitative model for the coupled kinetics of As adsorption/desorption/oxidation on δ-MnO2. The kinetics of As(III) adsorption and oxidation on δ-MnO2 was studied using a stirred-flow method under varying influent As(III) concentrations. The model was able to account for the variations in As concentration, different binding behavior of As(III) and As(V), and flow rates. Our model quantitatively shows that the coupled As adsorption/desorption/oxidation processes are highly dependent on As mass loading rates on δ-MnO2. Our model suggests that the As(III) adsorption rate is much higher than its oxidation rate. Both As(III) oxidation and subsequent As(V) desorption may proceed with similar reaction rates and control the overall reaction rates, although As(V) desorption rates may be affected by As(V) re-adsorption. Our kinetic model contributes to prediction of the dynamic behavior of As when manganese oxides are present.
中文翻译:

砷在锰氧化物上的吸附/解吸和氧化耦合动力学的定量模型
预测在氧化锰-水溶液界面处发生的砷(As)耦合动力学反应的能力对于全球范围内被砷污染的场所的管理和修复至关重要。对砷氧化与吸附/解吸动力学之间耦合的定量理解将使我们能够更准确地预测砷的动力学行为。在这项研究中,我们开发了一种新的定量模型作为吸附/解吸/氧化对δ-MnO的耦合动力学2。作为(III)的吸附和氧化对动力学δ-的MnO 2在不同的进水As(III)浓度下,使用搅拌流方法研究了H2O。该模型能够说明As浓度,As(III)和As(V)的不同结合行为以及流速的变化。我们的模型定量表明,联接作为吸附/解吸/氧化过程是高度依赖于δ-的MnO作为质量负载率2。我们的模型表明,As(III)的吸附速率远高于其氧化速率。尽管As(V)的解吸速率可能会受到As(V)的再吸附的影响,但As(III)的氧化和随后的As(V)的解吸都可以以相似的反应速率进行并控制总体反应速率。当存在锰氧化物时,我们的动力学模型有助于预测As的动力学行为。
更新日期:2018-03-06
中文翻译:

砷在锰氧化物上的吸附/解吸和氧化耦合动力学的定量模型
预测在氧化锰-水溶液界面处发生的砷(As)耦合动力学反应的能力对于全球范围内被砷污染的场所的管理和修复至关重要。对砷氧化与吸附/解吸动力学之间耦合的定量理解将使我们能够更准确地预测砷的动力学行为。在这项研究中,我们开发了一种新的定量模型作为吸附/解吸/氧化对δ-MnO的耦合动力学2。作为(III)的吸附和氧化对动力学δ-的MnO 2在不同的进水As(III)浓度下,使用搅拌流方法研究了H2O。该模型能够说明As浓度,As(III)和As(V)的不同结合行为以及流速的变化。我们的模型定量表明,联接作为吸附/解吸/氧化过程是高度依赖于δ-的MnO作为质量负载率2。我们的模型表明,As(III)的吸附速率远高于其氧化速率。尽管As(V)的解吸速率可能会受到As(V)的再吸附的影响,但As(III)的氧化和随后的As(V)的解吸都可以以相似的反应速率进行并控制总体反应速率。当存在锰氧化物时,我们的动力学模型有助于预测As的动力学行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号