当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enthalpic Driving Force for the Selective Absorption of CO2 by an Ionic Liquid
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acs.jpclett.8b00347 Clyde A. Daly 1 , Thomas Brinzer 2, 3 , Cecelia Allison 1 , Sean Garrett-Roe 2, 3 , Steven A. Corcelli 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acs.jpclett.8b00347 Clyde A. Daly 1 , Thomas Brinzer 2, 3 , Cecelia Allison 1 , Sean Garrett-Roe 2, 3 , Steven A. Corcelli 1
Affiliation
|
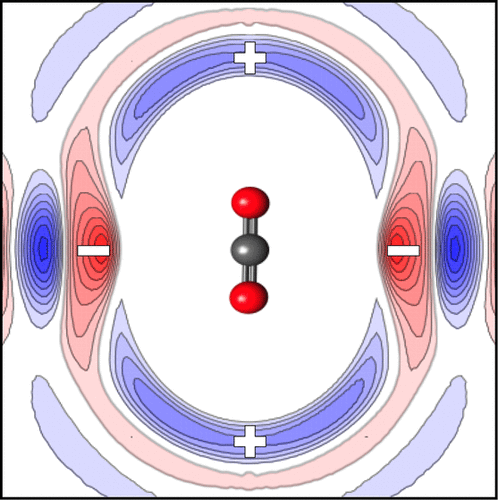
Molecular dynamics (MD) simulations validated against two-dimensional infrared (2D-IR) measurements of CO2 in an imidazolium-based ionic liquid have revealed new insights into the mechanism of CO2 solvation. The first solvation shell around CO2 has a distinctly quadrupolar structure, with strong negative charge density around the CO2 carbon atom and positive charge density near the CO2 oxygen atoms. When CO2 is modeled without atomic charges (thus removing its strong quadrupole moment), its solvation shell weakens and changes significantly into a structure that is similar to that of N2 in the same liquid. The solvation shell of CO2 evolves more quickly when its quadrupole is removed, and we find evidence that solvent cage dynamics is measured by 2D-IR spectroscopy. We also find that the solvent cage evolution of N2 is similar to that of CO2 with no atomic charges, implying that the weaker quadrupole of N2 is responsible for its higher diffusion and lower absorption in ionic liquids.
中文翻译:

离子液体选择性吸收CO 2的焓驱动力
针对基于咪唑鎓离子液体的CO 2的二维红外(2D-IR)测量验证的分子动力学(MD)模拟,揭示了对CO 2溶剂化机理的新见解。围绕CO所述第一溶剂化壳2有一个明显的四极结构,与周围的CO强的负电荷密度2碳原子和正电荷密度的CO附近2个氧原子。如果在没有原子电荷的情况下对CO 2进行建模(从而消除了其强大的四极矩),则其溶剂化壳会减弱并显着改变为类似于同一液体中N 2的结构。CO 2的溶剂化壳除去四极杆后,其演化更快,我们发现有证据表明溶剂笼动力学是通过2D-IR光谱法测量的。我们还发现,N 2的溶剂笼演化与无原子电荷的CO 2相似,这表明N 2的四极分子较弱是其在离子液体中的较高扩散和较低吸收的原因。
更新日期:2018-03-05
中文翻译:

离子液体选择性吸收CO 2的焓驱动力
针对基于咪唑鎓离子液体的CO 2的二维红外(2D-IR)测量验证的分子动力学(MD)模拟,揭示了对CO 2溶剂化机理的新见解。围绕CO所述第一溶剂化壳2有一个明显的四极结构,与周围的CO强的负电荷密度2碳原子和正电荷密度的CO附近2个氧原子。如果在没有原子电荷的情况下对CO 2进行建模(从而消除了其强大的四极矩),则其溶剂化壳会减弱并显着改变为类似于同一液体中N 2的结构。CO 2的溶剂化壳除去四极杆后,其演化更快,我们发现有证据表明溶剂笼动力学是通过2D-IR光谱法测量的。我们还发现,N 2的溶剂笼演化与无原子电荷的CO 2相似,这表明N 2的四极分子较弱是其在离子液体中的较高扩散和较低吸收的原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号