当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel‐Catalyzed Tandem Knoevenagel Condensation and Intramolecular Direct Arylation: Synthesis of Pyrazolo[5,1‐a]‐isoquinoline Derivatives
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-03-23 , DOI: 10.1002/adsc.201701519 Shiv Dhiman 1 , Nitesh Kumar Nandwana 1 , Hitesh Kumar Saini 1 , Dalip Kumar 1 , Krishnan Rangan 2 , Katherine N. Robertson 3 , Mukund Jha 4 , Anil Kumar 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-03-23 , DOI: 10.1002/adsc.201701519 Shiv Dhiman 1 , Nitesh Kumar Nandwana 1 , Hitesh Kumar Saini 1 , Dalip Kumar 1 , Krishnan Rangan 2 , Katherine N. Robertson 3 , Mukund Jha 4 , Anil Kumar 1
Affiliation
|
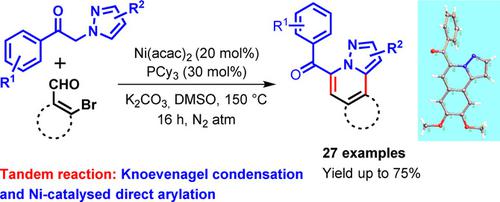
A simple and efficient method for the synthesis of pyrazolo[5,1‐a]isoquinoline derivatives has been developed using the nickel‐catalyzed reaction of 1‐aryl‐2‐(1H‐pyrazol‐1‐yl)ethan‐1‐ones and 2‐bromo aldehydes. The overall transformation involves tandem Knoevenagel condensation and intramolecular direct arylation via activation of the C5−H bond of the pyrazole ring. A series of 27 drug‐like aroyl−substituted pyrazolo[5,1‐a]isoquinolines has been synthesized in moderate to good yields.
中文翻译:

镍催化串联Knoevenagel缩合和分子内直接芳基化:吡唑并[5,1-a]-异喹啉衍生物的合成
使用1-芳基-2-(1 H-吡唑-1-基)乙烷-1-酮的镍催化反应,开发了一种简单高效的吡唑并[5,1- a ]异喹啉衍生物的合成方法。和2-溴醛。整个转化过程包括串联的Knoevenagel缩合和通过吡唑环的C5-H键活化而进行的分子内直接芳基化。合成了27种药物样的芳酰基取代的吡唑并[5,1- a ]异喹啉,收率中等至良好。
更新日期:2018-03-23
中文翻译:

镍催化串联Knoevenagel缩合和分子内直接芳基化:吡唑并[5,1-a]-异喹啉衍生物的合成
使用1-芳基-2-(1 H-吡唑-1-基)乙烷-1-酮的镍催化反应,开发了一种简单高效的吡唑并[5,1- a ]异喹啉衍生物的合成方法。和2-溴醛。整个转化过程包括串联的Knoevenagel缩合和通过吡唑环的C5-H键活化而进行的分子内直接芳基化。合成了27种药物样的芳酰基取代的吡唑并[5,1- a ]异喹啉,收率中等至良好。











































 京公网安备 11010802027423号
京公网安备 11010802027423号