当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The oxidation of borohydrides by photoexcited [UO2(CO3)3]4− †
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1039/c8dt00559a Koichiro Takao 1, 2, 3, 4, 5 , Satoru Tsushima 2, 3, 5, 6, 7
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1039/c8dt00559a Koichiro Takao 1, 2, 3, 4, 5 , Satoru Tsushima 2, 3, 5, 6, 7
Affiliation
|
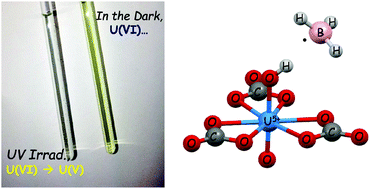
The carbonate ion is an effective quencher of uranyl(VI) luminescence and makes uranyl(VI) tricarbonate barely luminescent and photochemically inactive. We demonstrate here that photoexcited uranyl(VI) tricarbonate, *[UVIO2(CO3)3]4−, can however oxidize borohydrides (BH3X−, X = H, CN) to give boric acid and H2 gas, reducing itself to [UVO2(CO3)3]5−. This hypothesis was supported by UV-vis and NMR spectroscopy as well as quantum chemical calculations. The charge transfer states associated with photoreduction processes were modelled by density functional theory calculations. These results suggest that the mechanism of photoreduction of [UVIO2(CO3)3]4− is similar to that of [UVIO2(H2O)5]2+ and that it occurs through a one-photon reduction process.
中文翻译:

光激发[UO 2(CO 3)3 ] 4− †对硼氢化物的氧化
碳酸根离子是铀酰(VI)发光的有效猝灭剂,使铀酰(VI)三碳酸酯几乎不发光且无光化学活性。我们在这里表明,光激发双氧铀(VI)三碳酸,* [C VI ø 2(CO 3)3 ] 4-,但是可以氧化硼氢化物(BH 3 X -,X = H,CN),得到硼酸和H 2种气体,将自身还原为[U V O 2(CO 3)3 ] 5−。紫外可见光谱和NMR光谱以及量子化学计算支持了这一假设。通过密度泛函理论计算对与光还原过程相关的电荷转移状态进行建模。这些结果表明,[U VI O 2(CO 3)3 ] 4-的光还原机理与[U VI O 2(H 2 O)5 ] 2+相似,并且是通过单光子发生的。还原过程。
更新日期:2018-03-05
中文翻译:

光激发[UO 2(CO 3)3 ] 4− †对硼氢化物的氧化
碳酸根离子是铀酰(VI)发光的有效猝灭剂,使铀酰(VI)三碳酸酯几乎不发光且无光化学活性。我们在这里表明,光激发双氧铀(VI)三碳酸,* [C VI ø 2(CO 3)3 ] 4-,但是可以氧化硼氢化物(BH 3 X -,X = H,CN),得到硼酸和H 2种气体,将自身还原为[U V O 2(CO 3)3 ] 5−。紫外可见光谱和NMR光谱以及量子化学计算支持了这一假设。通过密度泛函理论计算对与光还原过程相关的电荷转移状态进行建模。这些结果表明,[U VI O 2(CO 3)3 ] 4-的光还原机理与[U VI O 2(H 2 O)5 ] 2+相似,并且是通过单光子发生的。还原过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号