当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, Antibacterial Activity and Molecular Docking of Substituted Naphthyridines as Potential DNA Gyrase Inhibitors
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-03-05 , DOI: 10.1002/slct.201800108 Farghaly A. Omar 1 , Mariam Abelrasoul 2 , Mahmoud M. Sheha 2 , Hoda Y. Hassan 2 , Yasser Musa. Ibrahiem 3
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-03-05 , DOI: 10.1002/slct.201800108 Farghaly A. Omar 1 , Mariam Abelrasoul 2 , Mahmoud M. Sheha 2 , Hoda Y. Hassan 2 , Yasser Musa. Ibrahiem 3
Affiliation
|
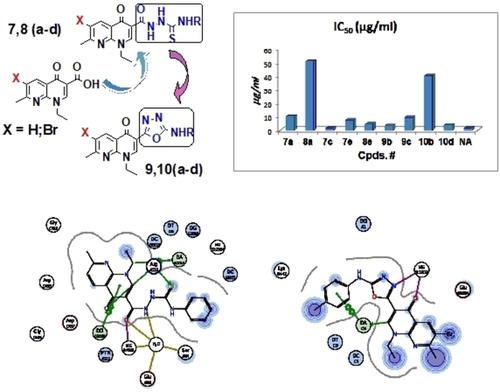
A series of naphthyridine‐3‐thiosemicarbazide 7,8(a–e) and the corresponding cyclized analogs, naphthyridine‐3‐(1,3,4‐oxadiazoles) 9,10(a–e) were synthesized through modification of the COOH in nalidixic acid (NA) and its 6‐bromo analogue, as new chemical entities (NCE) with enhanced antimicrobial potential. The compounds were screened for antibacterial activity against Gram positive (G+ve) strains (S. aureus, B. cereus); Gram negative (G‐ve) (E. coli, K. pneumonia, P. aeruginosa) and Mycobac. smegmatis. Compounds 7 b,c and 9 b,d displayed the highest activity against S. aureus (minimal inhibitory concentration; MIC ∼ 6–7 mM), whereas B. cereus was found to be more susceptible to the brominated oxadiazoles 10 b,d,e (MIC∼ 5.5‐5.9 mM). Moreover, 10 b,c,d exhibit similar MIC values against K. pneumonia and M. smegmatis. This demonstrates that bromination of the naphtyridone skeleton results in broader spectrum and enhanced antibacterial profile. In addition, the aryl substituted thioemicarbazides 7 c,d,e showed inhibitory effect of the growth of M. smegmatis at MIC ∼ 5.4‐7.1 mM. Molecular docking to DNA‐gyrase cleavage complex of S. aureus, Mycobac. (mTB) and Top. IV of K. pneumonia revealed similar binding poses to the co‐crystallized quinolone ligands and indicate good correlation of the binding energy (ΔG) with the observed MIC values of the active compounds. Consequently, DNA‐gyrase assay was proposed and executed. Most prominent DNA‐gyrase inhibition showed by the naphthyridinyl‐3‐thiosemicarbazides, 7 c and 8 e (IC50: 1.73 and 4.46 μg/mL respectively); and the oxadiazoles 9 b and 10 d (IC50: 3.36 and 3.89 μg/mL respectively). Assessment of drug‐likeness characteristics illustrates that the synthesized compounds showed agreement to Lipinsiki's and Veper's parameters. The study could offer an exceptional framework that may lead to the discovery of new potent antimicrobial agents.
中文翻译:

取代的萘啶类化合物作为潜在的DNA促旋酶抑制剂的合成,抗菌活性和分子对接
通过修饰COOH合成了一系列的萘啶-3-硫代氨基脲7,8(a-e)和相应的环化类似物萘吡啶-3-(1,3,4-恶二唑)9,10(a-e)。萘啶酸(NA)及其6-溴代类似物中的一种,具有增强的抗菌潜力的新化学实体(NCE)。筛选化合物对革兰氏阳性(G + ve)菌株(金黄色葡萄球菌,蜡状芽孢杆菌)的抗菌活性。革兰氏阴性(G-ve)(大肠杆菌,肺炎克雷伯菌,铜绿假单胞菌)和霉菌。耻垢分枝杆菌。化合物7 b,c和9 b,d针对显示最高活性的金黄色葡萄球菌(最小抑制浓度; MIC〜6-7 毫米),而蜡状芽孢杆菌被发现是与溴化二唑更敏感10b中,d,E(MIC〜5.5-5.9 毫米)。此外,10b,c,d对肺炎克雷伯菌和耻垢分枝杆菌显示出相似的MIC值。这表明萘啶酮骨架的溴化导致更宽的光谱和增强的抗菌性。另外,芳基取代的硫代氨基脲7 c,d,e表现出对生长的抑制作用。耻垢分枝杆菌的MIC约为5.4-7.1 mM。分子对接于DNA-促旋酶的裂解复杂的金黄色葡萄球菌,Mycobac。(mTB)和顶部。IV的肺炎克雷伯菌显示相似的结合构成了对共结晶的喹诺酮配体和表示结合能(的良好的相关性ΔG与观察到)MIC的活性化合物的值。因此,提出并执行了DNA回转酶测定法。萘吡啶-3-硫代氨基脲在7 c和8 e上表现出最显着的DNA旋转酶抑制作用(IC 50:分别为1.73和4.46μg/ mL);和恶二唑9b和10 d(IC 50分别为3.36和3.89μg / mL)。药物相似性特征的评估表明,合成的化合物显示出与Lipinsiki和Veper参数一致。该研究可能会提供一个例外的框架,从而可能导致发现新的有效抗菌剂。
更新日期:2018-03-05
中文翻译:

取代的萘啶类化合物作为潜在的DNA促旋酶抑制剂的合成,抗菌活性和分子对接
通过修饰COOH合成了一系列的萘啶-3-硫代氨基脲7,8(a-e)和相应的环化类似物萘吡啶-3-(1,3,4-恶二唑)9,10(a-e)。萘啶酸(NA)及其6-溴代类似物中的一种,具有增强的抗菌潜力的新化学实体(NCE)。筛选化合物对革兰氏阳性(G + ve)菌株(金黄色葡萄球菌,蜡状芽孢杆菌)的抗菌活性。革兰氏阴性(G-ve)(大肠杆菌,肺炎克雷伯菌,铜绿假单胞菌)和霉菌。耻垢分枝杆菌。化合物7 b,c和9 b,d针对显示最高活性的金黄色葡萄球菌(最小抑制浓度; MIC〜6-7 毫米),而蜡状芽孢杆菌被发现是与溴化二唑更敏感10b中,d,E(MIC〜5.5-5.9 毫米)。此外,10b,c,d对肺炎克雷伯菌和耻垢分枝杆菌显示出相似的MIC值。这表明萘啶酮骨架的溴化导致更宽的光谱和增强的抗菌性。另外,芳基取代的硫代氨基脲7 c,d,e表现出对生长的抑制作用。耻垢分枝杆菌的MIC约为5.4-7.1 mM。分子对接于DNA-促旋酶的裂解复杂的金黄色葡萄球菌,Mycobac。(mTB)和顶部。IV的肺炎克雷伯菌显示相似的结合构成了对共结晶的喹诺酮配体和表示结合能(的良好的相关性ΔG与观察到)MIC的活性化合物的值。因此,提出并执行了DNA回转酶测定法。萘吡啶-3-硫代氨基脲在7 c和8 e上表现出最显着的DNA旋转酶抑制作用(IC 50:分别为1.73和4.46μg/ mL);和恶二唑9b和10 d(IC 50分别为3.36和3.89μg / mL)。药物相似性特征的评估表明,合成的化合物显示出与Lipinsiki和Veper参数一致。该研究可能会提供一个例外的框架,从而可能导致发现新的有效抗菌剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号