当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rethinking Co(CO3)0.5(OH)·0.11H2O: a new property for highly selective electrochemical reduction of carbon dioxide to methanol in aqueous solution
Green Chemistry ( IF 9.3 ) Pub Date : 2018-03-02 , DOI: 10.1039/c7gc03744a Jianzhi Huang 1, 2, 3, 4, 5 , Qiong Hu 1, 2, 3, 4, 5 , Xinrong Guo 1, 2, 3, 4, 5 , Qiang Zeng 1, 2, 3, 4, 5 , Lishi Wang 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2018-03-02 , DOI: 10.1039/c7gc03744a Jianzhi Huang 1, 2, 3, 4, 5 , Qiong Hu 1, 2, 3, 4, 5 , Xinrong Guo 1, 2, 3, 4, 5 , Qiang Zeng 1, 2, 3, 4, 5 , Lishi Wang 1, 2, 3, 4, 5
Affiliation
|
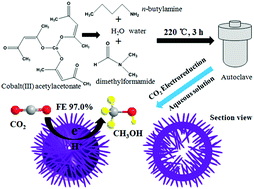
Co(CO3)0.5(OH)·0.11H2O is usually acknowledged and used as a precursor to synthesize other nanomaterials. However, some important properties of Co(CO3)0.5(OH)·0.11H2O have not been discovered yet. Herein, we report an important new property of hollow urchin-like Co(CO3)0.5(OH)·0.11H2O for highly selective electrochemical reduction of carbon dioxide to methanol in NaHCO3 aqueous solution at −0.98 V versus saturated calomel electrode (SCE) with Faradaic efficiency of up to 97.0% under ambient conditions, which is superior to most of the electrocatalysts reported to date. Finally, this low-cost electrocatalyst shows great potential in CO2 conversion industry for practical application in the future.
中文翻译:

对Co(CO 3)0.5(OH)·0.11H 2 O的重新思考:水溶液中二氧化碳高选择性电化学还原为甲醇的新性质
通常公认Co(CO 3)0.5(OH)·0.11H 2 O,并用作合成其他纳米材料的前体。但是,尚未发现Co(CO 3)0.5(OH)·0.11H 2 O的一些重要性质。在此,我们报告了空心顽固状的Co(CO 3)0.5(OH)·0.11H 2 O与在-0.98 V下NaHCO 3水溶液中的二氧化碳高选择性电化学还原成甲醇相比的重要新性能。饱和甘汞电极(SCE),在环境条件下的法拉第效率高达97.0%,优于迄今为止报道的大多数电催化剂。最后,这种低成本的电催化剂在CO 2转化工业中显示出巨大的潜力,可在将来实际应用。
更新日期:2018-07-02
中文翻译:

对Co(CO 3)0.5(OH)·0.11H 2 O的重新思考:水溶液中二氧化碳高选择性电化学还原为甲醇的新性质
通常公认Co(CO 3)0.5(OH)·0.11H 2 O,并用作合成其他纳米材料的前体。但是,尚未发现Co(CO 3)0.5(OH)·0.11H 2 O的一些重要性质。在此,我们报告了空心顽固状的Co(CO 3)0.5(OH)·0.11H 2 O与在-0.98 V下NaHCO 3水溶液中的二氧化碳高选择性电化学还原成甲醇相比的重要新性能。饱和甘汞电极(SCE),在环境条件下的法拉第效率高达97.0%,优于迄今为止报道的大多数电催化剂。最后,这种低成本的电催化剂在CO 2转化工业中显示出巨大的潜力,可在将来实际应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号