当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Thermodynamic description of Tc(iv) solubility and carbonate complexation in alkaline NaHCO3–Na2CO3–NaCl systems†
Dalton Transactions ( IF 4 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1039/c8dt00250a A. Baumann 1, 2, 3, 4 , E. Yalçıntaş 1, 2, 3, 4, 5 , X. Gaona 1, 2, 3, 4 , R. Polly 1, 2, 3, 4 , K. Dardenne 1, 2, 3, 4 , T. Prüßmann 1, 2, 3, 4 , J. Rothe 1, 2, 3, 4 , M. Altmaier 1, 2, 3, 4 , H. Geckeis 1, 2, 3, 4
Dalton Transactions ( IF 4 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1039/c8dt00250a A. Baumann 1, 2, 3, 4 , E. Yalçıntaş 1, 2, 3, 4, 5 , X. Gaona 1, 2, 3, 4 , R. Polly 1, 2, 3, 4 , K. Dardenne 1, 2, 3, 4 , T. Prüßmann 1, 2, 3, 4 , J. Rothe 1, 2, 3, 4 , M. Altmaier 1, 2, 3, 4 , H. Geckeis 1, 2, 3, 4
Affiliation
|
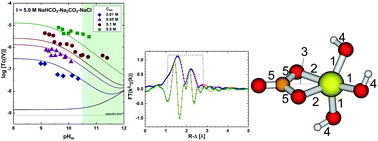
The solubility of 99Tc(IV) was investigated in dilute to concentrated carbonate solutions (0.01 M ≤ Ctot ≤ 1.0 M, with Ctot = [HCO3−] + [CO32−]) under systematic variation of ionic strength (I = 0.3–5.0 M NaHCO3–Na2CO3–NaCl–NaOH) and pHm (−log[H+] = 8.5–14.5). Strongly reducing conditions (pe + pHm ≈ 2) were set with Sn(II). Carbonate enhances the solubility of Tc(IV) in alkaline conditions by up to 3.5 log10-units compared to carbonate-free systems. Solvent extraction and XANES confirmed that Tc was kept as +IV during the timeframe of the experiments (≤ 650 days). Solid phase characterization performed by XAFS, XRD, SEM-EDS, chemical analysis and TG-DTA confirmed that TcO2·0.6H2O(am) controls the solubility of Tc(IV) under the conditions investigated. Slope analysis of the solubility data in combination with solid/aqueous phase characterization and DFT calculations indicate the predominance of the species Tc(OH)3CO3− at pHm ≤ 11 and Ctot ≥ 0.01 M, for which thermodynamic and activity models are derived. Solubility data obtained above pHm ≈ 11 indicates the formation of previously unreported Tc(IV)–carbonate species, possibly Tc(OH)4CO32−, although the likely formation of additional complexes prevents deriving a thermodynamic model valid for this pHm-region. This work provides the most comprehensive thermodynamic dataset available for the system Tc4+–Na+–Cl−–OH−–HCO3−–CO32−–H2O(l) valid under a range of conditions relevant for nuclear waste disposal.
中文翻译:

碱性NaHCO 3 -Na 2 CO 3 -NaCl体系 中Tc(iv)溶解度和碳酸盐络合的热力学描述†
的溶解度99 TC(IV)在稀进行了研究,以浓缩的碳酸盐溶液(0.01M≤Ç地合计≤1.0M,以C TOT = [HCO 3 - ] + [CO 3 2- ])下的离子强度的系统性变化(I = 0.3–5.0 M NaHCO 3 –Na 2 CO 3 –NaCl–NaOH)和pH m(-log [H + ] = 8.5-14.5)。强还原条件下(PE + pH值米≈2)与锡(设置II)。碳酸盐可提高Tc(IV)在碱性条件下的溶解度,最高可达3.5 log 10-单位与无碳酸盐的系统相比。溶剂萃取和XANES证实,在实验期间(≤650天),Tc保持为+ IV。通过XAFS,XRD,SEM-EDS,化学分析和TG-DTA进行的固相表征证实,在研究的条件下,TcO 2 ·0.6H 2 O(am)控制Tc(IV)的溶解度。与固相/水相的表征和DFT计算组合中的溶解度数据的斜率分析指示物种TC(OH)的优势3 CO 3 -在pH米≤11和C地合计≥0.01μm时,对于其中的热力学和活动模型是衍生的。在pH以上获得的溶解度数据米≈11指示以前未报告的TC(形成IV) -碳酸酯物种,可能TC(OH) 4 CO 3 2-,虽然附加的复合物防止了可能形成导出热力学模型有效该pH米-region。这项工作为系统Tc 4+ –Na + –Cl – –OH – –HCO 3 – –CO 3 2– –H 2 O(l)提供了最全面的热力学数据集,在与核废料有关的一系列条件下有效处理。
更新日期:2018-03-02
中文翻译:

碱性NaHCO 3 -Na 2 CO 3 -NaCl体系 中Tc(iv)溶解度和碳酸盐络合的热力学描述†
的溶解度99 TC(IV)在稀进行了研究,以浓缩的碳酸盐溶液(0.01M≤Ç地合计≤1.0M,以C TOT = [HCO 3 - ] + [CO 3 2- ])下的离子强度的系统性变化(I = 0.3–5.0 M NaHCO 3 –Na 2 CO 3 –NaCl–NaOH)和pH m(-log [H + ] = 8.5-14.5)。强还原条件下(PE + pH值米≈2)与锡(设置II)。碳酸盐可提高Tc(IV)在碱性条件下的溶解度,最高可达3.5 log 10-单位与无碳酸盐的系统相比。溶剂萃取和XANES证实,在实验期间(≤650天),Tc保持为+ IV。通过XAFS,XRD,SEM-EDS,化学分析和TG-DTA进行的固相表征证实,在研究的条件下,TcO 2 ·0.6H 2 O(am)控制Tc(IV)的溶解度。与固相/水相的表征和DFT计算组合中的溶解度数据的斜率分析指示物种TC(OH)的优势3 CO 3 -在pH米≤11和C地合计≥0.01μm时,对于其中的热力学和活动模型是衍生的。在pH以上获得的溶解度数据米≈11指示以前未报告的TC(形成IV) -碳酸酯物种,可能TC(OH) 4 CO 3 2-,虽然附加的复合物防止了可能形成导出热力学模型有效该pH米-region。这项工作为系统Tc 4+ –Na + –Cl – –OH – –HCO 3 – –CO 3 2– –H 2 O(l)提供了最全面的热力学数据集,在与核废料有关的一系列条件下有效处理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号