当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Shuttling Rates, Electronic States, and Hysteresis in a Ring-in-Ring Rotaxane
ACS Central Science ( IF 18.2 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acscentsci.7b00535 Mark C. Lipke 1 , Yilei Wu 2 , Indranil Roy 2 , Yuping Wang 2 , Michael R. Wasielewski 2 , J. Fraser Stoddart 2
ACS Central Science ( IF 18.2 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acscentsci.7b00535 Mark C. Lipke 1 , Yilei Wu 2 , Indranil Roy 2 , Yuping Wang 2 , Michael R. Wasielewski 2 , J. Fraser Stoddart 2
Affiliation
|
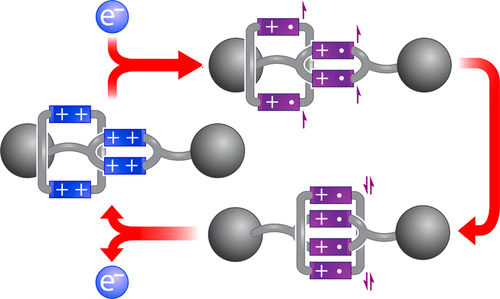
The trisradical recognition motif between a 4,4′-bipyridinium radical cation and a cyclo-bis-4,4′-bipyridinium diradical dication has been employed previously in rotaxanes to control their nanomechanical and electronic properties. Herein, we describe the synthesis and characterization of a redox-active ring-in-ring [2]rotaxane BBR·8PF6 that employs a tetraradical variant of this recognition motif. A square-shaped bis-4,4′-bipyridinium cyclophane is mechanically interlocked around the dumbbell component of this rotaxane, and the dumbbell itself incorporates a smaller bis-4,4′-bipyridinium cyclophane into its covalently bonded structure. This small cyclophane serves as a significant impediment to the shuttling of the larger ring across the dumbbell component of BBR8+, whereas reduction to the tetraradical tetracationic state BBR4(+•) results in strong association of the two cyclophanes driven by two radical-pairing interactions. In these respects, BBR·8PF6 exhibits qualitatively similar behavior to its predecessors that interconvert between hexacationic and trisradical tricationic states. The rigid preorganization of two bipyridinium groups within the dumbbell of BBR·8PF6 confers, however, two distinct properties upon this rotaxane: (1) the rate of shuttling is reduced significantly relative to those of its predecessors, resulting in marked electrochemical hysteresis observed by cyclic voltammetry for switching between the BBR8+/BBR4(+•) states, and (2) the formally tetraradical form of the rotaxane, BBR4(+•), exhibits a diamagnetic ground state, which, as a result of the slow shuttling motions within BBR4(+•), has a long enough lifetime to be characterized by 1H NMR spectroscopy.
中文翻译:

环式轮烷中的穿梭速率,电子状态和迟滞
在4,4'-联吡啶自由基阳离子和环-双-4,4'-联吡啶双自由基指示之间的三自由基识别基序先前已用于轮烷中以控制其纳米机械和电子性质。在这里,我们描述了采用该识别基序的四价变体的氧化还原活性环中[2]轮烷BBR· 8PF 6的合成和表征。正方形的bis-4,4'-联吡啶鎓环庚烷机械地互锁在该轮烷的哑铃组分周围,哑铃本身将较小的bis-4,4'-联吡啶鎓环庚烷掺入其共价键结构中。这种小的环烯显着阻碍了BBR哑铃组件上较大环的穿梭8+,而还原为四自由基四阳离子态BBR 4(+•)则导致两个环配对之间由两个自由基配对相互作用驱动的强环缔合。在这些方面, BBR ·8PF 6在质量上与其前身在六阳离子和三自由基三阳离子状态之间相互转换的行为相似。在BBR ·8PF 6的哑铃中,两个联吡啶基团的刚性预组织赋予了轮状烷烃两个不同的特性:(1)梭动速率相对于其前代显着降低,从而导致明显的电化学滞后现象。循环伏安法在BBR之间切换8+ / BBR 4(+•)态,以及(2)轮烷的正式四自由基形式BBR 4(+•)表现出反磁性基态,这是由于BBR 4( +•)具有足够长的寿命,可通过1 H NMR光谱表征。
更新日期:2018-03-02
中文翻译:

环式轮烷中的穿梭速率,电子状态和迟滞
在4,4'-联吡啶自由基阳离子和环-双-4,4'-联吡啶双自由基指示之间的三自由基识别基序先前已用于轮烷中以控制其纳米机械和电子性质。在这里,我们描述了采用该识别基序的四价变体的氧化还原活性环中[2]轮烷BBR· 8PF 6的合成和表征。正方形的bis-4,4'-联吡啶鎓环庚烷机械地互锁在该轮烷的哑铃组分周围,哑铃本身将较小的bis-4,4'-联吡啶鎓环庚烷掺入其共价键结构中。这种小的环烯显着阻碍了BBR哑铃组件上较大环的穿梭8+,而还原为四自由基四阳离子态BBR 4(+•)则导致两个环配对之间由两个自由基配对相互作用驱动的强环缔合。在这些方面, BBR ·8PF 6在质量上与其前身在六阳离子和三自由基三阳离子状态之间相互转换的行为相似。在BBR ·8PF 6的哑铃中,两个联吡啶基团的刚性预组织赋予了轮状烷烃两个不同的特性:(1)梭动速率相对于其前代显着降低,从而导致明显的电化学滞后现象。循环伏安法在BBR之间切换8+ / BBR 4(+•)态,以及(2)轮烷的正式四自由基形式BBR 4(+•)表现出反磁性基态,这是由于BBR 4( +•)具有足够长的寿命,可通过1 H NMR光谱表征。



























 京公网安备 11010802027423号
京公网安备 11010802027423号