当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pH-Dependent Surface Chemistry from First Principles: Application to the BiVO4(010)–Water Interface
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acsami.7b16545 Francesco Ambrosio 1 , Julia Wiktor 1 , Alfredo Pasquarello 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acsami.7b16545 Francesco Ambrosio 1 , Julia Wiktor 1 , Alfredo Pasquarello 1
Affiliation
|
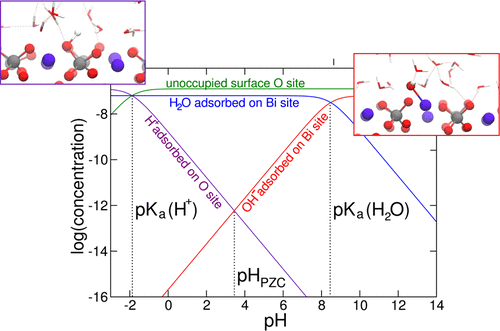
We present a theoretical formulation for studying the pH-dependent interfacial coverage of semiconductor–water interfaces through ab initio electronic structure calculations, molecular dynamics simulations, and the thermodynamic integration method. This general methodology allows one to calculate the acidity of the individual adsorption sites on the surface and consequently the pH at the point of zero charge, pHPZC, and the preferential adsorption mode of water molecules, either molecular or dissociative, at the semiconductor–water interface. The proposed method is applied to study the BiVO4(010)–water interface, yields a pHPZC in excellent agreement with the experimental characterization. Furthermore, from the calculated pKa values of the individual adsorption sites, we construct an ab initio concentration diagram of all adsorbed species at the interface as a function of the pH of the aqueous solution. The diagram clearly illustrates the pH-dependent coverage of the surface and indicates that protons are found to be significantly adsorbed (∼1% of available sites) only in highly acidic conditions. The surface is found to be mostly covered by molecularly adsorbed water molecules in a wide interval of pH values ranging from 2 to 8. Hydroxyl ions are identified as the dominant adsorbed species at pH larger than 8.2.
中文翻译:

第一性原理从pH依赖的表面化学:在BiVO 4(010)-水界面中的应用
我们提供了一个理论公式,用于通过从头算电子结构计算,分子动力学模拟和热力学积分方法研究半导体与水界面的pH依赖性界面覆盖。这种通用方法允许人们计算表面上各个吸附位点的酸度,从而计算出零电荷时的pH值pH PZC以及半导体水上分子或解离性水分子的优先吸附模式。界面。所提出的方法用于研究BiVO 4(010)-水的界面,产生的pH PZC与实验特性非常吻合。此外,根据计算出的p K a各个吸附位的值,我们构建了界面处所有吸附物质的从头算浓度图,该浓度是水溶液pH的函数。该图清楚地说明了表面的pH依赖性覆盖,并表明仅在强酸性条件下,质子才被吸附(约占可用位点的1%)。发现在2至8的宽pH值范围内,该表面大部分被分子吸附的水分子覆盖。在大于8.2的pH值下,羟基离子被认为是主要的吸附物质。
更新日期:2018-03-02
中文翻译:

第一性原理从pH依赖的表面化学:在BiVO 4(010)-水界面中的应用
我们提供了一个理论公式,用于通过从头算电子结构计算,分子动力学模拟和热力学积分方法研究半导体与水界面的pH依赖性界面覆盖。这种通用方法允许人们计算表面上各个吸附位点的酸度,从而计算出零电荷时的pH值pH PZC以及半导体水上分子或解离性水分子的优先吸附模式。界面。所提出的方法用于研究BiVO 4(010)-水的界面,产生的pH PZC与实验特性非常吻合。此外,根据计算出的p K a各个吸附位的值,我们构建了界面处所有吸附物质的从头算浓度图,该浓度是水溶液pH的函数。该图清楚地说明了表面的pH依赖性覆盖,并表明仅在强酸性条件下,质子才被吸附(约占可用位点的1%)。发现在2至8的宽pH值范围内,该表面大部分被分子吸附的水分子覆盖。在大于8.2的pH值下,羟基离子被认为是主要的吸附物质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号