当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A General Synthetic Route to Polycyclic Aromatic Dicarboximides by Palladium-Catalyzed Annulation Reaction
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acs.joc.8b00301 Kazutaka Shoyama 1 , Magnus Mahl 1 , Sabine Seifert 1 , Frank Würthner 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acs.joc.8b00301 Kazutaka Shoyama 1 , Magnus Mahl 1 , Sabine Seifert 1 , Frank Würthner 1, 2
Affiliation
|
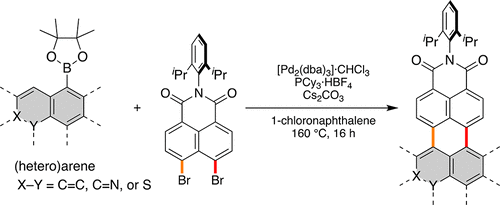
Here we report a general method for the synthesis of polycyclic aromatic dicarboximides (PADIs) by palladium-catalyzed annulation of naphthalene dicarboximide to different types of aromatic substrates. Reaction conditions were optimized by systematic variation of ligand, solvent, and additive. It was shown that solvent has a decisive effect on the yield of the reaction products, and thus 1-chloronaphthalene as solvent afforded the highest yield. By applying the optimized reaction conditions, a broad series of planar carbo- and heterocycle containing PADIs were synthesized in up to 97% yield. Moreover, this approach could be applied to curved aromatic scaffold to achieve the respective bowl-shaped PADI. Two-fold annulation was accomplished by employing arene diboronic esters, affording polycyclic aromatic bis(dicarboximides). The optical and electrochemical properties of this broad series of PADIs were explored as well.
中文翻译:

钯催化环化反应合成多环芳族二甲酰亚胺的一般合成路线
在这里,我们报告了通过钯催化的萘二甲酰亚胺向不同类型的芳香族底物的合成来合成多环芳族二甲酰亚胺(PADI)的一般方法。通过配体,溶剂和添加剂的系统变化来优化反应条件。结果表明,溶剂对反应产物的收率具有决定性的影响,因此1-氯萘作为溶剂收率最高。通过应用优化的反应条件,合成了一系列平面含碳和杂环的PADI,收率高达97%。而且,该方法可以应用于弯曲的芳族支架以实现相应的碗形PADI。通过使用芳烃二硼酸酯完成两次环化反应,得到多环芳族双(二芳基二酰亚胺)。
更新日期:2018-03-02
中文翻译:

钯催化环化反应合成多环芳族二甲酰亚胺的一般合成路线
在这里,我们报告了通过钯催化的萘二甲酰亚胺向不同类型的芳香族底物的合成来合成多环芳族二甲酰亚胺(PADI)的一般方法。通过配体,溶剂和添加剂的系统变化来优化反应条件。结果表明,溶剂对反应产物的收率具有决定性的影响,因此1-氯萘作为溶剂收率最高。通过应用优化的反应条件,合成了一系列平面含碳和杂环的PADI,收率高达97%。而且,该方法可以应用于弯曲的芳族支架以实现相应的碗形PADI。通过使用芳烃二硼酸酯完成两次环化反应,得到多环芳族双(二芳基二酰亚胺)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号