Biomaterials ( IF 12.8 ) Pub Date : 2018-03-02 , DOI: 10.1016/j.biomaterials.2018.02.052 Emily Wonder 1 , Lorena Simón-Gracia 2 , Pablo Scodeller 3 , Ramsey N Majzoub 1 , Venkata Ramana Kotamraju 4 , Kai K Ewert 1 , Tambet Teesalu 5 , Cyrus R Safinya 1
|
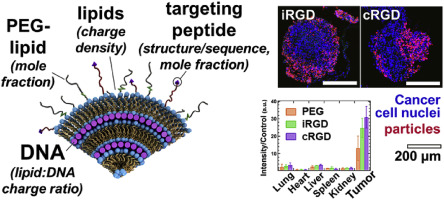
Cationic liposome–nucleic acid (CL–NA) complexes, which form spontaneously, are a highly modular gene delivery system. These complexes can be sterically stabilized via PEGylation [PEG: poly (ethylene glycol)] into nanoparticles (NPs) and targeted to specific tissues and cell types via the conjugation of an affinity ligand. However, there are currently no guidelines on how to effectively navigate the large space of compositional parameters that modulate the specific and nonspecific binding interactions of peptide-targeted NPs with cells. Such guidelines are desirable to accelerate the optimization of formulations with novel peptides. Using PEG-lipids functionalized with a library of prototypical tumor-homing peptides, we varied the peptide density and other parameters (binding motif, peptide charge, CL/DNA charge ratio) to study their effect on the binding and uptake of the corresponding NPs. We used flow cytometry to quantitatively assess binding as well as internalization of NPs by cultured cancer cells. Surprisingly, full peptide coverage resulted in less binding and internalization than intermediate coverage, with the optimum coverage varying between cell lines. In, addition, our data revealed that great care must be taken to prevent nonspecific electrostatic interactions from interfering with the desired specific binding and internalization. Importantly, such considerations must take into account the charge of the peptide ligand as well as the membrane charge density and the CL/DNA charge ratio. To test our guidelines, we evaluated the in vivo tumor selectivity of selected NP formulations in a mouse model of peritoneally disseminated human gastric cancer. Intraperitoneally administered peptide-tagged CL–DNA NPs showed tumor binding, minimal accumulation in healthy control tissues, and preferential penetration of smaller tumor nodules, a highly clinically relevant target known to drive recurrence of the peritoneal cancer.
中文翻译:

肽标记的阳离子脂质体-DNA纳米粒子在体外和体内的电荷介导和特异性结合的竞争
自发形成的阳离子脂质体-核酸(CL-NA)复合物是一种高度模块化的基因传递系统。这些复合物可以通过聚乙二醇化[PEG:聚乙二醇]实现空间稳定,形成纳米颗粒(NP),并通过亲和配体的缀合靶向特定的组织和细胞类型。然而,目前还没有关于如何有效操纵大范围的成分参数来调节肽靶向纳米粒子与细胞的特异性和非特异性结合相互作用的指南。这些指南对于加速新型肽制剂的优化是可取的。使用带有原型肿瘤归巢肽库功能化的 PEG-脂质,我们改变了肽密度和其他参数(结合基序、肽电荷、CL/DNA 电荷比),以研究它们对相应 NP 的结合和摄取的影响。我们使用流式细胞术定量评估培养癌细胞对纳米粒子的结合和内化。令人惊讶的是,完全肽覆盖导致比中间覆盖更少的结合和内化,并且最佳覆盖在细胞系之间有所不同。此外,我们的数据表明,必须非常小心,以防止非特异性静电相互作用干扰所需的特异性结合和内化。重要的是,此类考虑必须考虑肽配体的电荷以及膜电荷密度和 CL/DNA 电荷比。为了测试我们的指南,我们在腹膜播散性人类胃癌小鼠模型中评估了选定的 NP 制剂的体内肿瘤选择性。 腹腔内施用肽标记的 CL-DNA NPs 显示出肿瘤结合性、在健康对照组织中的最小积累以及较小肿瘤结节的优先渗透,这是已知可驱动腹膜癌复发的高度临床相关的目标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号