当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Benzylic Allylic Alkylation Reaction of 3‐Furfural Derivatives by Dearomatizative Dienamine Activation
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-04-06 , DOI: 10.1002/chem.201800585 Xiao-Long He 1 , Hui-Ru Zhao 1 , Chuan-Qi Duan 1 , Xu Han 1 , Wei Du 1 , Ying-Chun Chen 1, 2
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-04-06 , DOI: 10.1002/chem.201800585 Xiao-Long He 1 , Hui-Ru Zhao 1 , Chuan-Qi Duan 1 , Xu Han 1 , Wei Du 1 , Ying-Chun Chen 1, 2
Affiliation
|
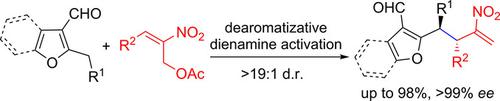
The dearomatizative dienamine‐type ortho‐quinodimethane species are smoothly generated between 2‐alkyl‐3‐furfurals and chiral secondary amine catalysts, which undergo asymmetric benzylic allylic alkylation reactions with 2‐nitroallylic acetates efficiently. A spectrum of densely functionalized 3‐furfural derivatives are delivered in moderate to high yields with good to excellent diastereo‐ and enantioselectivity (up to 98 % yield, >19:1 d.r., >99 % ee). The latent transformations allow the facile production of some enantioenriched architectures, such as 1,1,2,2‐tetraarylethanes and triarylmethanes, which are not easily available from other protocols.
中文翻译:

脱芳香化二烯胺活化的3-糠醛衍生物的不对称苯甲酸烯丙基烷基化反应
脱芳香化的二烯胺型邻喹啉甲烷物质在2-烷基-3-糠醛和手性仲胺催化剂之间平稳生成,它们与2-硝基烯丙基乙酸盐有效地进行了不对称的苄基烯丙基烷基化反应。一系列功能强大的3-糠醛衍生物以中等至高收率提供,具有良好或优异的非对映选择性和对映选择性(最高收率98%,> 19:1 dr,> 99% ee)。潜在的转化使得可以轻松生产一些对映体富集的体系结构,例如1,1,2,2-四芳基乙烷和三芳基甲烷,这是其他协议中不容易获得的。
更新日期:2018-04-06
中文翻译:

脱芳香化二烯胺活化的3-糠醛衍生物的不对称苯甲酸烯丙基烷基化反应
脱芳香化的二烯胺型邻喹啉甲烷物质在2-烷基-3-糠醛和手性仲胺催化剂之间平稳生成,它们与2-硝基烯丙基乙酸盐有效地进行了不对称的苄基烯丙基烷基化反应。一系列功能强大的3-糠醛衍生物以中等至高收率提供,具有良好或优异的非对映选择性和对映选择性(最高收率98%,> 19:1 dr,> 99% ee)。潜在的转化使得可以轻松生产一些对映体富集的体系结构,例如1,1,2,2-四芳基乙烷和三芳基甲烷,这是其他协议中不容易获得的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号