Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2018-03-02 , DOI: 10.1016/j.jinorgbio.2018.02.016 Valerio Ferrario , Niels Hansen , Jürgen Pleiss
|
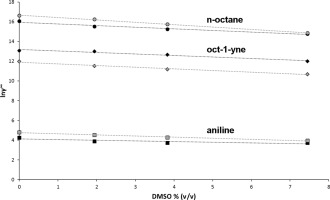
The experimentally determined Michaelis constant Kmc results from a combination of two effects: the recognition of the substrate by the enzyme and the molecular interactions between substrate and solvent. By separating substrate recognition from solvent effects, the thermodynamic activity-based Michaelis constant Kma allows for an unambiguous comparison of how different substrates fit into the substrate binding site. Kma of a poorly water-soluble substrate is calculated from the experimentally determined concentration-based Michaelis constant Kmc and its activity coefficient at infinite dilution γ∞. Comparing the Kma of different substrates instead of the experimentally determined Kmc prevents misinterpretations of the molecular basis of enzyme-substrate interactions. While n-octane showed the lowest Kmc value of six P450BM-3 substrates, its Kma was 500 fold higher than aniline, indicating that the binding of n-octane is mainly driven by its low water solubility, while binding of aniline is driven by its shape complementarity. For three substrates (aniline, oct-1-yne, n-octane), γ∞ was reliably calculated by molecular dynamics simulations, either in binary substrate-water mixtures or in ternary mixtures including DMSO as cosolvent. It is demonstrated that the widely used DMSO has a considerable effect on the measured Kmc value. Depending on the substrate, addition of 10% v/v DMSO increases Kmc by up to a factor of 11. To make biocatalytic experiments reproducible, it is therefore of utmost importance to carefully report the reaction conditions. The reliable simulation of activity coefficients in complex mixtures allows for an unambiguous comparison of enzyme-substrate interactions and provides a predictive tool for the design of biocatalytic processes.
中文翻译:

通过模拟热力学活性解释细胞色素P450单加氧酶的动力学
实验确定的米氏常数K m c是两种作用的组合:酶对底物的识别以及底物与溶剂之间的分子相互作用。通过将底物识别与溶剂作用分开,基于热力学活性的米氏常数K m a可以明确比较不同底物如何适合底物结合位点。ķ米一个一个水难溶性基板的从实验确定的基于浓度的米氏常数计算ķ米Ç和其活性系数在无限稀释γ ∞。比较不同底物的K m a而不是实验确定的K m c可以防止对酶与底物相互作用的分子基础的误解。尽管正辛烷值是六种P450 BM-3底物的最低K m c值,但其K m a比苯胺高500倍,这表明正辛烷值的结合主要是由其低水溶性引起的,而正辛烷值的结合力却最大。苯胺是由其形状互补性驱动的。对于三种底物(苯胺,辛-1-炔,正辛烷),γ∞通过分子动力学模拟,可以可靠地计算出二元底物-水混合物或三元混合物(包括DMSO作为助溶剂)中的H2O3。事实证明,广泛使用的DMSO对测得的K m c值有相当大的影响。根据底物的不同,添加10%v / v DMSO可使K m c最多提高11倍。为了使生物催化实验具有可重现性,因此仔细报告反应条件至关重要。复杂混合物中活度系数的可靠模拟可以明确比较酶与底物之间的相互作用,并为生物催化过程的设计提供了预测工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号