Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-03-02 , DOI: 10.1016/j.actbio.2018.02.021 Erik V Munsell 1 , Deepa S Kurpad 2 , Theresa A Freeman 2 , Millicent O Sullivan 1
|
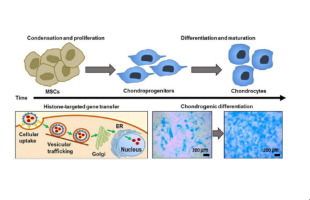
Skeletal tissue regeneration following traumatic injury involves a complex cascade of growth factor signals that direct the differentiation of mesenchymal stem cells (MSCs) within the fracture. The necessity for controlled and localized expression of these factors has highlighted the role gene therapy may play as a promising treatment option for bone repair. However, the design of nanocarrier systems that negotiate efficient intracellular trafficking and nuclear delivery represents a significant challenge. Recent investigations have highlighted the roles histone tail sequences play in directing nuclear delivery and activating DNA transcription. We previously established the ability to recapitulate these natural histone tail activities within non-viral nanocarriers, improving gene transfer and expression by enabling effective navigation to the nucleus via retrograde vesicular trafficking. Herein, we demonstrate that histone-targeting leads to ∼4-fold enhancements in osteogenic bone morphogenetic protein-2 (BMP-2) expression by MSCs over 6 days, as compared with standard polymeric transfection reagents. This improved expression augmented chondrogenesis, an essential first step in fracture healing. Importantly, significant enhancements of cartilage-specific protein expression were triggered by histone-targeted gene transfer, as compared with the response to treatment with equivalent amounts of recombinant BMP-2 protein. In fact, a 100-fold increase in recombinant BMP-2 was required to achieve similar levels of chondrogenic gene and protein expression. The enhancements in differentiation achieved using histone-targeting were in part enabled by an increase in transcription factor expression, which functioned to drive MSC chondrogenesis. These novel findings demonstrate the utility of histone-targeted gene transfer strategies to enable substantial reductions in BMP-2 dosing for bone regenerative applications.
Statement of Significance
This contribution addresses significant limitations in non-viral gene transfer for bone regenerative applications by exploiting a novel histone-targeting approach for cell-triggered delivery that induces osteogenic BMP-2 expression coincident with the initiation of bone repair. During repair, proliferating mesenchymal stem cells (MSCs) respond to a complex series of growth factor signals that direct their differentiation along cellular lineages essential to mature bone formation. Although these MSCs are ideal targets for enhanced transfection during cellular mitosis, few non-viral delivery approaches exist to enable maximization of this effect. Accordingly, this contribution seeks to utilize our histone-targeted nanocarrier design strategy to stimulate BMP-2 gene transfer in dividing MSCs. This gene-based approach leads to significantly augmented MSC chondrogenesis, an essential first step in bone tissue repair.
中文翻译:

骨形态发生蛋白 2 的组蛋白靶向基因转移可增强间充质干细胞软骨形成分化。
创伤性损伤后的骨骼组织再生涉及一系列复杂的生长因子信号级联,这些信号指导骨折内的间充质干细胞(MSC)的分化。这些因子受控和局部表达的必要性凸显了基因治疗作为骨修复的一种有前景的治疗选择的作用。然而,协调有效的细胞内运输和核递送的纳米载体系统的设计是一个重大挑战。最近的研究强调了组蛋白尾序列在指导核递送和激活 DNA 转录中的作用。我们之前建立了在非病毒纳米载体中重现这些天然组蛋白尾部活性的能力,通过逆行囊泡运输有效导航至细胞核,从而改善基因转移和表达。在此,我们证明,与标准聚合转染试剂相比,组蛋白靶向导致 MSC 在 6 天内的成骨骨形态发生蛋白 2 (BMP-2) 表达增强约 4 倍。这种改善的表达增强了软骨形成,这是骨折愈合的重要第一步。重要的是,与等量重组 BMP-2 蛋白治疗的反应相比,组蛋白靶向基因转移引发了软骨特异性蛋白表达的显着增强。事实上,需要将重组 BMP-2 增加 100 倍才能达到类似水平的软骨形成基因和蛋白质表达。使用组蛋白靶向实现的分化增强部分是由于转录因子表达的增加而实现的,转录因子表达的作用是驱动 MSC 软骨形成。 这些新发现证明了组蛋白靶向基因转移策略的实用性,可以大幅减少骨再生应用中 BMP-2 的剂量。
重要性声明
这项贡献通过利用一种新的组蛋白靶向方法进行细胞触发递送,在骨修复启动的同时诱导成骨 BMP-2 表达,解决了骨再生应用中非病毒基因转移的重大局限性。在修复过程中,增殖的间充质干细胞 (MSC) 对一系列复杂的生长因子信号做出反应,这些信号引导它们沿着成熟骨形成所必需的细胞谱系分化。尽管这些 MSC 是细胞有丝分裂过程中增强转染的理想靶标,但很少有非病毒递送方法可以最大限度地发挥这种效果。因此,本贡献旨在利用我们的组蛋白靶向纳米载体设计策略来刺激分裂 MSC 中的 BMP-2 基因转移。这种基于基因的方法可显着增强 MSC 软骨形成,这是骨组织修复的重要第一步。











































 京公网安备 11010802027423号
京公网安备 11010802027423号