Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-03-01 , DOI: 10.1016/j.actbio.2018.02.020 Bo Huang 1 , Walter L Siqueira 2 , Dennis G Cvitkovitch 1 , Yoav Finer 1
|
Objectives
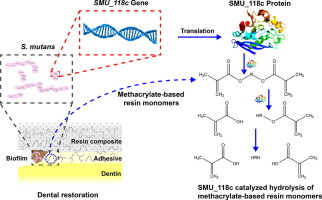
To identify and characterize specific esterases from S. mutans with degradative activity toward methacrylate-based resin monomers.
Methods
Out of several putative esterases, an esterase encoded in an Open Reading Frame as SMU_118c (The National Center for Biotechnology Information, NCBI), was found to have true hydrolase activities. SMU_118c was cloned, expressed, purified and further characterized for its respective hydrolytic activity towards ester-containing nitrophenyl substrates and the universal resin monomers bis-phenyl-glycidyl-dimethacrylate (bisGMA) and triethyleneglycol dimethacrylate (TEGDMA) at neutral (7.0) or cariogenic (5.5) pH. Mass spectrometry (MS) was used to verify the expression of SMU_118c protein in S. mutans UA159.
Results
Similar to the whole cell activity of S. mutans, SMU_118c showed the highest affinity toward p-nitrophenyl acetate (pNPA) and p-nitrophenyl butyrate (pNPB) vs. o-nitrophenyl butyrate (oNPB) and butyrylthiocholine iodide (BTC) (p<0.05). The esterase retained 60% of its activity after 21 days and hydrolyzed bisGMA at a higher rate than TEGDMA at both neutral and cariogenic pH (p<0.001), similarly to the predominant human salivary esterase degradative activity. MS confirmed that SMU_118c is an intracellular protein in S. mutans UA159 and expressed under pathogenic (pH 5.5) growth conditions.
Significance
The similarity in the activity profile to the whole S. mutans bacterial cell, the stability over time at cariogenic pH, the preference to hydrolyze bisGMA and confirmed expression profile suggest that SMU_118c could be a significant contributor to the whole bacterial degradative activity of S. mutans toward the degradation of resin composites, adhesives and the restoration-tooth interface, potentially accelerating restoration’s failure.
Statement of Significance
The current study builds upon our highly-cited previous study by Bourbia et al., (JDR, 2013) that reported on that the cariogenic bacterium, S. mutans has esterase-like activities that enable the bacteria to degrade dental composites and adhesives. The current submission is the first to report on the isolation and characterization of the specific esterase activity (SMU_118c) from S. mutans that is a significant contributor to the whole bacterial degradative activity toward the hydrolysis of dental resins. This activity compromises the restoration-tooth interface, increase interfacial bacterial microleakage (Kermanshahi et al., JDR 2010), potentially contributing to the pathogenesis of recurrent caries around resin composite restorations. This represent a significant contribution to the field of biomaterials and their clinical performance.
中文翻译:

来自致龋细菌的酯酶可水解牙科树脂。
目标
鉴定和表征来自变形链球菌的对甲基丙烯酸酯树脂单体具有降解活性的特定酯酶。
方法
在几种假定的酯酶中,一种在开放阅读框架中编码为 SMU_118c(国家生物技术信息中心,NCBI)的酯酶被发现具有真正的水解酶活性。 SMU_118c 被克隆、表达、纯化并进一步表征其在中性 (7.0) 或致龋性 (7.0) 下对含酯硝基苯基底物和通用树脂单体二甲基丙烯酸二苯基缩水甘油酯 (bisGMA) 和二甲基丙烯酸三甘醇酯 (TEGDMA) 各自的水解活性。 5.5)pH值。使用质谱(MS)验证SMU_118c蛋白在变形链球菌UA159中的表达。
结果
与变形链球菌的全细胞活性相似, SMU_118c 对乙酸对硝基苯酯 (pNPA) 和丁酸对硝基苯酯 (pNPB) 与丁酸邻硝基苯酯 (oNPB) 和碘化丁酰硫胆碱 (BTC)相比表现出最高的亲和力 (p< 0 id=77>S.变形菌UA159并在致病性(pH 5.5)生长条件下表达。
意义
与整个变形链球菌细菌细胞的活性谱的相似性、在致龋性 pH 下随时间的稳定性、水解 bisGMA 的偏好以及经证实的表达谱表明 SMU_118c 可能是变形链球菌整个细菌降解活性的重要贡献者树脂复合材料、粘合剂和修复体-牙齿界面的降解,可能会加速修复体的失败。
重要性声明
目前的研究建立在 Bourbia 等人先前被高度引用的研究(JDR,2013)的基础上,该研究报告了致龋细菌S. mutans具有类似酯酶的活性,使细菌能够降解牙科复合材料和粘合剂。目前提交的材料是第一个报告来自变形链球菌的特定酯酶活性 (SMU_118c) 的分离和表征,该活性对整个细菌对牙科树脂水解的降解活性有重要贡献。这种活性会损害修复体-牙齿界面,增加界面细菌微渗漏(Kermanshahi 等人,JDR 2010),可能导致树脂复合修复体周围复发性龋齿的发病机制。这代表了对生物材料领域及其临床表现的重大贡献。











































 京公网安备 11010802027423号
京公网安备 11010802027423号