当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Flipped Phenyl Ring Orientations of Dopamine Binding with Human and Drosophila Dopamine Transporters: Remarkable Role of Three Nonconserved Residues
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-03-01 00:00:00 , DOI: 10.1021/acschemneuro.8b00030 Yaxia Yuan 1 , Jun Zhu 2 , Chang-Guo Zhan 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-03-01 00:00:00 , DOI: 10.1021/acschemneuro.8b00030 Yaxia Yuan 1 , Jun Zhu 2 , Chang-Guo Zhan 1
Affiliation
|
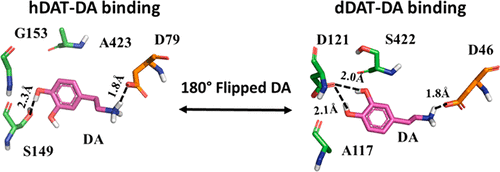
Molecular modeling and molecular dynamics simulations were performed in the present study to examine the modes of dopamine binding with human and Drosophila dopamine transporters (hDAT and dDAT). The computational data revealed flipped binding orientations of dopamine in hDAT and dDAT due to the major differences in three key residues (S149, G153, and A423 of hDAT vs A117, D121, and S422 of dDAT) in the binding pocket. These three residues dictate the binding orientation of dopamine in the binding pocket, as the aromatic ring of dopamine tends to take an orientation with both the para- and meta-hydroxyl groups being close to polar residues and away from nonpolar residues of the protein. The flipped binding orientations of dopamine in hDAT and dDAT clearly demonstrate a generally valuable insight concerning how the species difference could drastically affect the protein–ligand binding modes, demonstrating that the species difference, which is a factor rarely considered in early drug design stage, must be accounted for throughout the ligand/drug design and discovery processes in general.
中文翻译:

与人和果蝇多巴胺转运蛋白结合的多巴胺的翻转苯环取向:三种非保守残基的显着作用。
在本研究中进行了分子建模和分子动力学模拟,以检验多巴胺与人和果蝇结合的模式。多巴胺转运蛋白(hDAT和dDAT)。计算数据显示,由于结合口袋中三个关键残基(hDAT的S149,G153和A423与dDAT的A117,D121和S422)的主要差异,hDAT和dDAT中多巴胺的结合方向发生了翻转。这三个残基决定了多巴胺在结合口袋中的结合方向,因为多巴胺的芳香环倾向于以对羟基和间羟基均靠近极性残基且远离蛋白质的非极性残基的方式取向。多巴胺在hDAT和dDAT中的结合方向翻转,清楚地表明了关于物种差异如何极大地影响蛋白质-配体结合模式的普遍有价值的见解,表明物种差异是药物设计早期很少考虑的一个因素,
更新日期:2018-03-01
中文翻译:

与人和果蝇多巴胺转运蛋白结合的多巴胺的翻转苯环取向:三种非保守残基的显着作用。
在本研究中进行了分子建模和分子动力学模拟,以检验多巴胺与人和果蝇结合的模式。多巴胺转运蛋白(hDAT和dDAT)。计算数据显示,由于结合口袋中三个关键残基(hDAT的S149,G153和A423与dDAT的A117,D121和S422)的主要差异,hDAT和dDAT中多巴胺的结合方向发生了翻转。这三个残基决定了多巴胺在结合口袋中的结合方向,因为多巴胺的芳香环倾向于以对羟基和间羟基均靠近极性残基且远离蛋白质的非极性残基的方式取向。多巴胺在hDAT和dDAT中的结合方向翻转,清楚地表明了关于物种差异如何极大地影响蛋白质-配体结合模式的普遍有价值的见解,表明物种差异是药物设计早期很少考虑的一个因素,











































 京公网安备 11010802027423号
京公网安备 11010802027423号