当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Layered LiTiO2 for the protection of Li2S cathodes against dissolution: mechanisms of the remarkable performance boost†
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1039/c8ee00419f Feixiang Wu 1, 2, 3, 4 , Travis P. Pollard 4, 5, 6, 7 , Enbo Zhao 1, 2, 3, 4 , Yiran Xiao 1, 2, 3, 4 , Marco Olguin 4, 5, 6, 7 , Oleg Borodin 4, 5, 6, 7 , Gleb Yushin 1, 2, 3, 4
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1039/c8ee00419f Feixiang Wu 1, 2, 3, 4 , Travis P. Pollard 4, 5, 6, 7 , Enbo Zhao 1, 2, 3, 4 , Yiran Xiao 1, 2, 3, 4 , Marco Olguin 4, 5, 6, 7 , Oleg Borodin 4, 5, 6, 7 , Gleb Yushin 1, 2, 3, 4
Affiliation
|
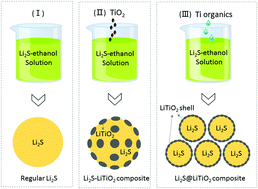
Lithium sulfide (Li2S) cathodes have been viewed as very promising candidates for next-generation lightweight Li and Li-ion batteries. Prior work on the deposition of carbon shells around Li2S particles showed reduced dissolution of polysulfides and improved cathode stability. However, due to the substantial volume changes during cycling and the low chemical binding energy between carbon and sulfides, defects almost inevitably forming in the carbon shell during battery operation commonly lead to premature cell failure. In this study, we show that conformal coatings of layered LiTiO2 may offer better protection against polysulfide dissolution and the shuttle effects. Density functional theory (DFT) calculations revealed that LiTiO2 exhibits a strong affinity for sulfur species (Li2Sx) and, most importantly, induces a rapid conversion of longer (highly soluble) polysulfides to short polysulfides, which exhibit minimum solubility in electrolytes. Quite remarkably, even the mere presence of the electronically conductive layered oxides (LiMO2, M = metal) such as LiTiO2 in the cathodes (e.g., as a component of the mix with Li2S) enhanced the cell rate and cycling stability dramatically. Advanced material characterization in combination with quantum chemistry calculations provided unique insights into the mechanisms of the incredible performance boost, such as interactions between Li2Sx and the LiTiO2 surface, leading to breakage of S–S bonds.
中文翻译:

用于保护Li 2 S阴极免于溶解的 层状LiTiO 2:显着提高性能的机制†
硫化锂(Li 2 S)阴极已被视为下一代轻质Li和Li离子电池的非常有前途的候选材料。先前关于在Li 2 S颗粒周围沉积碳壳的工作表明减少了多硫化物的溶解并改善了阴极稳定性。但是,由于循环过程中体积的巨大变化以及碳与硫化物之间的化学结合能低,电池操作期间几乎不可避免地会在碳壳中形成缺陷,这通常会导致电池过早失效。在这项研究中,我们表明层状LiTiO 2的保形涂层可以提供更好的保护,防止多硫化物溶解和穿梭效应。密度泛函理论(DFT)计算表明LiTiO 2对硫物质(Li 2 S x)具有很强的亲和力,最重要的是,可诱导较长(高度溶解)的多硫化物迅速转化为短的多硫化物,而短的多硫化物在电解质中的溶解度最低。非常显着的是,即使在阴极中仅存在导电层状氧化物(LiMO 2,M =金属),例如LiTiO 2(例如,作为与Li 2 S的混合物的一部分),也极大地提高了电池速率和循环稳定性。先进的材料表征与量子化学计算相结合,提供了对令人难以置信的性能提升机制(例如Li 2 S之间的相互作用)的独特见解x和LiTiO 2表面,导致S–S键断裂。
更新日期:2018-03-02
中文翻译:

用于保护Li 2 S阴极免于溶解的 层状LiTiO 2:显着提高性能的机制†
硫化锂(Li 2 S)阴极已被视为下一代轻质Li和Li离子电池的非常有前途的候选材料。先前关于在Li 2 S颗粒周围沉积碳壳的工作表明减少了多硫化物的溶解并改善了阴极稳定性。但是,由于循环过程中体积的巨大变化以及碳与硫化物之间的化学结合能低,电池操作期间几乎不可避免地会在碳壳中形成缺陷,这通常会导致电池过早失效。在这项研究中,我们表明层状LiTiO 2的保形涂层可以提供更好的保护,防止多硫化物溶解和穿梭效应。密度泛函理论(DFT)计算表明LiTiO 2对硫物质(Li 2 S x)具有很强的亲和力,最重要的是,可诱导较长(高度溶解)的多硫化物迅速转化为短的多硫化物,而短的多硫化物在电解质中的溶解度最低。非常显着的是,即使在阴极中仅存在导电层状氧化物(LiMO 2,M =金属),例如LiTiO 2(例如,作为与Li 2 S的混合物的一部分),也极大地提高了电池速率和循环稳定性。先进的材料表征与量子化学计算相结合,提供了对令人难以置信的性能提升机制(例如Li 2 S之间的相互作用)的独特见解x和LiTiO 2表面,导致S–S键断裂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号