当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Binding sites for luminescent amyloid biomarkers from non-biased molecular dynamics simulations
Chemical Communications ( IF 4.3 ) Pub Date : 2018-03-01 00:00:00 , DOI: 10.1039/c8cc00105g Carolin König 1, 2, 3, 4 , Robin Skånberg 4, 5, 6, 7 , Ingrid Hotz 4, 5, 6, 7 , Anders Ynnerman 4, 5, 6, 7 , Patrick Norman 1, 2, 3, 4 , Mathieu Linares 1, 2, 3, 4, 8
Chemical Communications ( IF 4.3 ) Pub Date : 2018-03-01 00:00:00 , DOI: 10.1039/c8cc00105g Carolin König 1, 2, 3, 4 , Robin Skånberg 4, 5, 6, 7 , Ingrid Hotz 4, 5, 6, 7 , Anders Ynnerman 4, 5, 6, 7 , Patrick Norman 1, 2, 3, 4 , Mathieu Linares 1, 2, 3, 4, 8
Affiliation
|
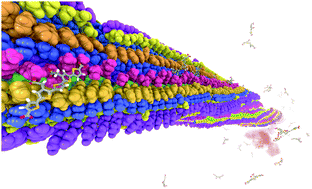
A very stable binding site for the interaction between a pentameric oligothiophene and an amyloid-β(1–42) fibril has been identified by means of non-biased molecular dynamics simulations. In this site, the probe is locked in an all-trans conformation with a Coulombic binding energy of 1200 kJ mol−1 due to the interactions between the anionic carboxyl groups of the probe and the cationic ε-amino groups in the lysine side chain. Upon binding, the conformationally restricted probes show a pronounced increase in molecular planarity. This is in line with the observed changes in luminescence properties that serve as the foundation for their use as biomarkers.
中文翻译:

来自无偏分子动力学模拟的发光淀粉样生物标志物的结合位点
通过无偏分子动力学模拟,已经确定了五聚体寡噻吩与淀粉样β(1-42)原纤维之间相互作用的非常稳定的结合位点。在该位置,由于探针的阴离子羧基与赖氨酸侧链中的阳离子ε-氨基之间的相互作用,探针以1200 kJ mol -1的库仑结合能被锁定为全反式构象。结合后,构象受限的探针显示出分子平面度的显着增加。这与所观察到的发光性质变化相一致,这些变化为它们用作生物标记物奠定了基础。
更新日期:2018-03-21
中文翻译:

来自无偏分子动力学模拟的发光淀粉样生物标志物的结合位点
通过无偏分子动力学模拟,已经确定了五聚体寡噻吩与淀粉样β(1-42)原纤维之间相互作用的非常稳定的结合位点。在该位置,由于探针的阴离子羧基与赖氨酸侧链中的阳离子ε-氨基之间的相互作用,探针以1200 kJ mol -1的库仑结合能被锁定为全反式构象。结合后,构象受限的探针显示出分子平面度的显着增加。这与所观察到的发光性质变化相一致,这些变化为它们用作生物标记物奠定了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号