当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst-Free Ring Opening of Spiroaziridine Oxindoles by Heteronucleophiles: An Approach to the Synthesis of Enantiopure 3-Substituted Oxindoles
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-01 00:00:00 , DOI: 10.1021/acs.joc.7b03288 Saumen Hajra 1 , Somnath Singha Roy 1 , Anurag Biswas 1 , Sk Abu Saleh 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-01 00:00:00 , DOI: 10.1021/acs.joc.7b03288 Saumen Hajra 1 , Somnath Singha Roy 1 , Anurag Biswas 1 , Sk Abu Saleh 1
Affiliation
|
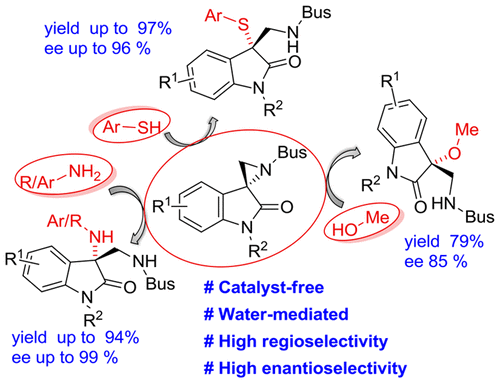
A simple catalyst-free method was developed for the ring opening of spiroaziridine oxindoles by three different nucleophiles, namely, amines, thiols, and methanol, to produce enantiopure (up to 99%) vicinal diaminooxindoles, β-aminosulfides, and β-amino-3-methoxyoxindole, respectively, in good to excellent yields. In contrast to the spiroepoxides, spiroaziridines are opened regio- and stereospecifically through the pseudobenzylic spirocenter under catalyst-free conditions. Moreover, unlike simple 2-substituted aziridines, these spiroaziridines are opened up with retention in configuration at the C3-spirocenter.
中文翻译:

杂环亲核试剂对螺氮丙啶氧化吲哚的无催化剂开环:对映体3-取代的氧化吲哚的合成方法
开发了一种简单的无催化剂方法,用于通过三种不同的亲核试剂(即胺,硫醇和甲醇)使螺环丙啶羟吲哚开环,以产生对映纯(最高99%)的邻位二氨基恶吲哚,β-氨基硫化物和β-氨基- 3-甲氧基氧吲哚分别以良好至优异的产率。与螺环氧化物相反,螺环氮丙啶在无催化剂的条件下通过假苄基螺中心在区域上和立体上开放。而且,与简单的2-取代的氮丙啶不同,这些螺氮丙啶在C3-螺中心保留了构型而被打开。
更新日期:2018-03-01
中文翻译:

杂环亲核试剂对螺氮丙啶氧化吲哚的无催化剂开环:对映体3-取代的氧化吲哚的合成方法
开发了一种简单的无催化剂方法,用于通过三种不同的亲核试剂(即胺,硫醇和甲醇)使螺环丙啶羟吲哚开环,以产生对映纯(最高99%)的邻位二氨基恶吲哚,β-氨基硫化物和β-氨基- 3-甲氧基氧吲哚分别以良好至优异的产率。与螺环氧化物相反,螺环氮丙啶在无催化剂的条件下通过假苄基螺中心在区域上和立体上开放。而且,与简单的2-取代的氮丙啶不同,这些螺氮丙啶在C3-螺中心保留了构型而被打开。











































 京公网安备 11010802027423号
京公网安备 11010802027423号