Catalysis Today ( IF 5.2 ) Pub Date : 2018-03-01 , DOI: 10.1016/j.cattod.2018.02.055 Xiao Jiang , Xiaoxing Wang , Xiaowa Nie , Naoto Koizumi , Xinwen Guo , Chunshan Song
|
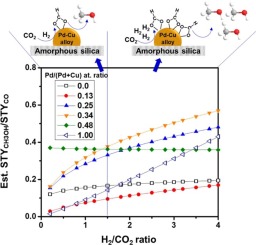
Our laboratory has observed a strong bimetallic promoting effect on SiO2-supported Pd-Cu catalysts for CH3OH formation from CO2 hydrogenation when the Pd/(Pd + Cu) atomic ratio lied within 0.25–0.34. In the present study, the activities of bimetallic and monometallic catalysts were comparatively evaluated at H2/CO2 = 1, and the origin for the bimetallic promoting effect was sought by a kinetic study and in situ DRIFTS analysis. The bimetallic promoting effect was retained at H2/CO2 = 1, although both the activity and CH3OH selectivity decreased, indicating the significant role of hydrogen partial pressure in the CO2 hydrogenation. Kinetic study provided insights into the strong dependence of CH3OH synthesis on H2/CO2 ratios and roles of Pd-Cu alloys in the observed CH3OH promotion, wherein the alloys could tune the surface sites balance of adsorbed species, and enabled the reduction of activation barrier for CH3OH synthesis. Temperature-programmed reduction results corroborated the strong interaction between Pd and Cu and its impact on the alloy structuring and reducibility. In situ DRIFTS analysis identified formate and carbonyl species were dominant on the surface during the reaction. The surface coverage of formate species was dependent on the catalyst composition, and appeared to correlate to the methanol promotion, implying its key role in the CH3OH synthesis on Pd-Cu catalysts.
中文翻译:

在Pd-Cu双金属催化剂上将CO 2加氢成甲醇:H 2 / CO 2的比例依赖性和表面种类
我们的实验室已经观察到,当Pd /(Pd + Cu)原子比在0.25-0.34范围内时,SiO 2负载的Pd-Cu催化剂对CO 2加氢形成CH 3 OH具有很强的双金属促进作用。在本研究中,在H 2 / CO 2 = 1的条件下,对双金属和单金属催化剂的活性进行了比较评估,并通过动力学研究和原位DRIFTS分析寻找了双金属促进作用的根源。 尽管活性和CH 3 OH选择性均降低,但双金属促进作用仍保持在H 2 / CO 2 = 1处,这表明氢分压在CO 2中的重要作用氢化。动力学研究提供了对CH 3 OH合成对H 2 / CO 2比的强烈依赖性以及Pd-Cu合金在观察到的CH 3 OH促进中的作用的深刻见解,其中合金可以调节吸附物种的表面位点平衡,并能够减少CH 3的激活势垒OH合成。程序升温还原结果证实了Pd和Cu之间的强相互作用及其对合金组织和还原性的影响。原位DRIFTS分析确定了甲酸和羰基物质在反应过程中占主导地位。甲酸盐类物质的表面覆盖率取决于催化剂的组成,并且似乎与甲醇的促进作用相关,这暗示了其在Pd-Cu催化剂上CH 3 OH合成中的关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号