当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Extraction Optimization and Structural and Thermal Characterization of the Antimicrobial Abietane 7α-Acetoxy-6β-hydroxyroyleanone
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-03-01 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00892 Carlos E. S. Bernardes 1 , Catarina Garcia 2, 3 , Filipe Pereira 2, 3 , Joana Mota 2 , P. Pereira 2, 4 , Maria J. Cebola 2, 5 , Catarina P. Reis 2, 6 , Isabel Correia 7 , M. Fátima M. Piedade 1, 7 , Manuel E. Minas da Piedade 1 , Patrícia Rijo 2, 6
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-03-01 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00892 Carlos E. S. Bernardes 1 , Catarina Garcia 2, 3 , Filipe Pereira 2, 3 , Joana Mota 2 , P. Pereira 2, 4 , Maria J. Cebola 2, 5 , Catarina P. Reis 2, 6 , Isabel Correia 7 , M. Fátima M. Piedade 1, 7 , Manuel E. Minas da Piedade 1 , Patrícia Rijo 2, 6
Affiliation
|
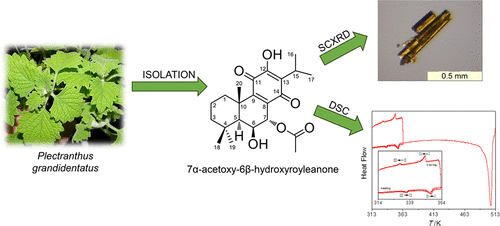
The abietane 7α-acetoxy-6β-hydroxyroyleanone (AHR), obtained from plant extracts, is an attractive lead for drug development, given its known antimicrobial properties. Two basic requirements to establish any compound as a new drug are the development of a convenient extraction process and the characterization of its structural and thermal properties. In this work seven different methods were tested to optimize the extraction of AHR from Plectranthus grandidentatus. Supercritical fluid extraction (SFE) proved to be the method of choice, delivering an amount of AHR (57.351 μg·mg–1) approximately six times higher than the second best method (maceration in acetone; 9.77 μg·mg–1). Single crystal X-ray diffraction analysis of the ARH molecular and crystal structure carried out at 167 ± 2 K and 296 ± 2 K showed only a single phase, here dubbed form III (orthorhombic space group P21212), at those temperatures. The presence of two other polymorphs above room temperature was, however, evidenced by differential scanning calorimetry (DSC). The three forms are enantiotropically related, with the form III → form II and form II → form I transitions occurring at 333.5 ± 1.6 K and 352.0 ± 1.6 K, respectively. The fact that the transitions are reversible suggests that polymorphism is not likely to be an issue in the development pharmaceutical formulations based on ARH. DSC experiments also showed that the compound decomposes on melting at 500.8 ± 0.8 K. Melting should therefore be avoided if, for example, strategies to improve solubility based on the production of glassy materials or solid dispersions are considered.
中文翻译:

抗菌剂Abietane7α-乙酰氧基-6β-羟基royleanone的提取工艺优化及结构和热性质
得自植物提取物的Abetanee7α-乙酰氧基-6β-羟基丁香酮(AHR),由于其已知的抗菌特性,因此是药物开发的诱人先导。建立任何化合物作为新药的两个基本要求是开发方便的提取工艺以及表征其结构和热性能。在这项工作中,测试了七种不同的方法以优化从侧柏中提取AHR的方法。超临界流体萃取(SFE)被证明是选择的方法,提供AHR的量(57.351微克·毫克-1)中比在丙酮第二最佳方法(浸渍大约六倍; 9.77微克·毫克-1)。在167±2 K和296±2 K上对ARH分子和晶体结构进行的单晶X射线衍射分析显示,只有一个单相,此处称为III型(斜方空间群P 2 1 2 12),在那些温度下。然而,通过差示扫描量热法(DSC)证明存在高于室温的另外两种多晶型物。这三种形式是对映体相关的,形式III→形式II和形式II→形式I的转变分别发生在333.5±1.6 K和352.0±1.6 K处。过渡是可逆的事实表明,在基于ARH的开发药物制剂中,多态性不太可能成为问题。DSC实验还表明,该化合物在500.8±0.8 K的熔点下分解。因此,例如,如果考虑基于玻璃状材料或固体分散体的生产来提高溶解度的策略,则应避免熔融。
更新日期:2018-03-01
中文翻译:

抗菌剂Abietane7α-乙酰氧基-6β-羟基royleanone的提取工艺优化及结构和热性质
得自植物提取物的Abetanee7α-乙酰氧基-6β-羟基丁香酮(AHR),由于其已知的抗菌特性,因此是药物开发的诱人先导。建立任何化合物作为新药的两个基本要求是开发方便的提取工艺以及表征其结构和热性能。在这项工作中,测试了七种不同的方法以优化从侧柏中提取AHR的方法。超临界流体萃取(SFE)被证明是选择的方法,提供AHR的量(57.351微克·毫克-1)中比在丙酮第二最佳方法(浸渍大约六倍; 9.77微克·毫克-1)。在167±2 K和296±2 K上对ARH分子和晶体结构进行的单晶X射线衍射分析显示,只有一个单相,此处称为III型(斜方空间群P 2 1 2 12),在那些温度下。然而,通过差示扫描量热法(DSC)证明存在高于室温的另外两种多晶型物。这三种形式是对映体相关的,形式III→形式II和形式II→形式I的转变分别发生在333.5±1.6 K和352.0±1.6 K处。过渡是可逆的事实表明,在基于ARH的开发药物制剂中,多态性不太可能成为问题。DSC实验还表明,该化合物在500.8±0.8 K的熔点下分解。因此,例如,如果考虑基于玻璃状材料或固体分散体的生产来提高溶解度的策略,则应避免熔融。











































 京公网安备 11010802027423号
京公网安备 11010802027423号