Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Critical Role of Water and Oxygen Defects in C–O Scission during CO2 Reduction on Zn2GeO4(010)
Langmuir ( IF 3.7 ) Pub Date : 2018-03-01 00:00:00 , DOI: 10.1021/acs.langmuir.7b03360 Jing Yang , Yanlu Li , Xian Zhao , Weiliu Fan
Langmuir ( IF 3.7 ) Pub Date : 2018-03-01 00:00:00 , DOI: 10.1021/acs.langmuir.7b03360 Jing Yang , Yanlu Li , Xian Zhao , Weiliu Fan
|
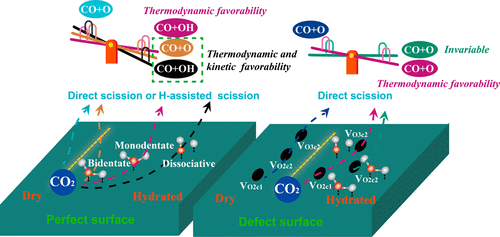
Exploration of catalyst structure and environmental sensitivity for C–O bond scission is essential for improving the conversion efficiency because of the inertness of CO2. We performed density functional theory calculations to understand the influence of the properties of adsorbed water and the reciprocal action with oxygen vacancy on the CO2 dissociation mechanism on Zn2GeO4(010). When a perfect surface was hydrated, the introduction of H2O was predicted to promote the scission step by two modes based on its appearance, with the greatest enhancement from dissociative adsorbed H2O. The dissociative H2O lowers the barrier and reaction energy of CO2 dissociation through hydrogen bonding to preactivate the C–O bond and assisted scission via a COOH intermediate. The perfect surface with bidentate-binding H2O was energetically more favorable for CO2 dissociation than the surface with monodentate-binding H2O. Direct dissociation was energetically favored by the former, whereas monodentate H2O facilitated the H-assisted pathway. The defective surface exhibited a higher reactivity for CO2 decomposition than the perfect surface because the generation of oxygen vacancies could disperse the product location. When the defective surface was hydrated, the reciprocal action for vacancy and surface H2O on CO2 dissociation was related to the vacancy type. The presence of H2O substantially decreased the reaction energy for the direct dissociation of CO2 on O2c1- and O3c2-defect surfaces, which converts the endoergic reaction to an exoergic reaction. However, the increased decomposition barrier made the step kinetically unfavorable and reduced the reaction rate. When H2O was present on the O2c2-defect surface, both the barrier and reaction energy for direct dissociation were invariable. This result indicated that the introduction of H2O had little effect on the kinetics and thermodynamics. Moreover, the H-assisted pathway was suppressed on all hydrated defect surfaces. These results provide a theoretical perspective for the design of highly efficient catalysts.
中文翻译:

Zn 2 GeO 4(010)上CO 2还原过程中水和氧缺陷在C–O断裂中的关键作用
由于CO 2的惰性,探索C–O键断裂的催化剂结构和环境敏感性对于提高转化效率至关重要。我们进行了密度泛函理论计算,以了解吸附水的性质以及氧空位的相互作用对Zn 2 GeO 4(010)的CO 2离解机理的影响。当完美的表面被水合时,根据其外观,H 2 O的引入可通过两种模式促进分裂过程,最大程度地促进了离解性吸附H 2 O的分解。离解性H 2 O降低了势垒和反应能的CO2通过氢键解离以预激活C–O键,并通过COOH中间体辅助断裂。具有二齿结合的H 2 O的理想表面比具有单齿结合的H 2 O的表面在能量上更有利于CO 2分解。前者在能量上有利于直接离解,而单齿H 2 O促进了H辅助途径。有缺陷的表面对CO 2的分解具有比完美表面更高的反应性,因为氧空位的产生会分散产物的位置。当缺陷表面被水化时,空位和表面H 2 O对CO 2的相互作用解离与空缺类型有关。H的存在2 ö基本上降低了反应能量为CO的直接离解2 O对2C1 -和O 3C2 -defect表面,其转换为放热反应的吸热反应。但是,增加的分解势垒使该步骤在动力学上不利,并降低了反应速率。当O 2c2缺陷表面上存在H 2 O时,直接离解的势垒和反应能均不变。该结果表明H 2的引入O对动力学和热力学影响很小。此外,在所有水合缺陷表面上均抑制了H辅助途径。这些结果为高效催化剂的设计提供了理论依据。
更新日期:2018-03-01
中文翻译:

Zn 2 GeO 4(010)上CO 2还原过程中水和氧缺陷在C–O断裂中的关键作用
由于CO 2的惰性,探索C–O键断裂的催化剂结构和环境敏感性对于提高转化效率至关重要。我们进行了密度泛函理论计算,以了解吸附水的性质以及氧空位的相互作用对Zn 2 GeO 4(010)的CO 2离解机理的影响。当完美的表面被水合时,根据其外观,H 2 O的引入可通过两种模式促进分裂过程,最大程度地促进了离解性吸附H 2 O的分解。离解性H 2 O降低了势垒和反应能的CO2通过氢键解离以预激活C–O键,并通过COOH中间体辅助断裂。具有二齿结合的H 2 O的理想表面比具有单齿结合的H 2 O的表面在能量上更有利于CO 2分解。前者在能量上有利于直接离解,而单齿H 2 O促进了H辅助途径。有缺陷的表面对CO 2的分解具有比完美表面更高的反应性,因为氧空位的产生会分散产物的位置。当缺陷表面被水化时,空位和表面H 2 O对CO 2的相互作用解离与空缺类型有关。H的存在2 ö基本上降低了反应能量为CO的直接离解2 O对2C1 -和O 3C2 -defect表面,其转换为放热反应的吸热反应。但是,增加的分解势垒使该步骤在动力学上不利,并降低了反应速率。当O 2c2缺陷表面上存在H 2 O时,直接离解的势垒和反应能均不变。该结果表明H 2的引入O对动力学和热力学影响很小。此外,在所有水合缺陷表面上均抑制了H辅助途径。这些结果为高效催化剂的设计提供了理论依据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号