Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2018-03-01 , DOI: 10.1016/j.jinorgbio.2018.02.013 Dipanwita Batabyal 1 , Thomas L Poulos 1
|
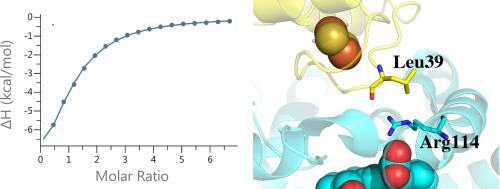
We have compared the thermodynamics of substrate and redox partner binding of P450cam to its close homologue, CYP101D1, using isothermal titration calorimetry (ITC). CYP101D1 binds camphor about 10-fold more weakly than P450cam which is consistent with the inability of camphor to cause a complete low- to high-spin shift in CYP101D1. Even so molecular dynamics simulations show that camphor is very stable in the CYP101D1 active site similar to P450cam. ITC data on the binding of the CYP101D1 ferredoxin redox partner (abbreviated Arx) shows that the substrate-bound closed state of CYP101D1 binds Arx more tightly than the substrate-free open form. This is just the opposite to P450cam where Pdx (ferredoxin redox partner of P450cam) favors binding to the P450cam open state. In addition, CYP101D1-Arx binding has a large negative ΔS while the P450cam-Pdx has a much smaller ΔS indicating that interactions at the docking interface are different. The most obvious difference is that PDXD38 which forms an important ion pair with P450camR112 at the center of the interface is ArxL39 in Arx. This suggests that Arx may adopt a different orientation than Pdx in order to optimize nonpolar interactions with ArxL39.
中文翻译:

氧化还原伙伴结合对 CYP101D1 构象动力学的影响
我们使用等温滴定量热法 (ITC) 比较了 P450cam 与其密切同源物 CYP101D1 的底物和氧化还原伴侣结合的热力学。CYP101D1 与樟脑的结合比 P450cam 弱约 10 倍,这与樟脑无法在 CYP101D1 中引起完全的低自旋到高自旋转变相一致。即便如此,分子动力学模拟表明,与 P450cam 类似,樟脑在 CYP101D1 活性位点上非常稳定。ITC 关于 CYP101D1 铁氧还蛋白氧化还原伴侣(缩写为 Arx)结合的数据表明,CYP101D1 与底物结合的闭合状态比无底物开放形式与 Arx 的结合更紧密。这与 P450cam 相反,其中 Pdx(P450cam 的铁氧还蛋白氧化还原伙伴)有利于与 P450cam 打开状态结合。此外,CYP101D1-Arx 结合具有较大的负 ΔS,而 P450cam-Pdx 具有小得多的 ΔS,表明对接界面处的相互作用不同。最明显的区别是在界面中心与P450cam R112形成重要离子对的PDX D38是Arx中的Arx L39。这表明 Arx 可能采用与 Pdx 不同的方向,以优化与 Arx L39的非极性相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号