当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lactate Racemase Nickel-Pincer Cofactor Operates by a Proton-Coupled Hydride Transfer Mechanism
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1021/acs.biochem.8b00100 Joel A. Rankin , Robert C. Mauban 1 , Matthias Fellner , Benoît Desguin 2 , John McCracken , Jian Hu , Sergey A. Varganov 1 , Robert P. Hausinger
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1021/acs.biochem.8b00100 Joel A. Rankin , Robert C. Mauban 1 , Matthias Fellner , Benoît Desguin 2 , John McCracken , Jian Hu , Sergey A. Varganov 1 , Robert P. Hausinger
Affiliation
|
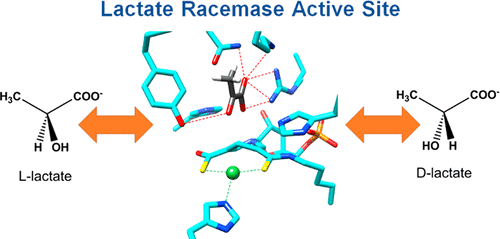
Lactate racemase (LarA) of Lactobacillus plantarum contains a novel organometallic cofactor with nickel coordinated to a covalently tethered pincer ligand, pyridinium-3-thioamide-5-thiocarboxylic acid mononucleotide, but its function in the enzyme mechanism has not been elucidated. This study presents direct evidence that the nickel-pincer cofactor facilitates a proton-coupled hydride transfer (PCHT) mechanism during LarA-catalyzed lactate racemization. No signal was detected by electron paramagnetic resonance spectroscopy for LarA in the absence or presence of substrate, consistent with a +2 metal oxidation state and inconsistent with a previously proposed proton-coupled electron transfer mechanism. Pyruvate, the predicted intermediate for a PCHT mechanism, was observed in quenched solutions of LarA. A normal substrate kinetic isotope effect (kH/kD of 3.11 ± 0.17) was established using 2-α-2H-lactate, further supporting a PCHT mechanism. UV–visible spectroscopy revealed a lactate-induced perturbation of the cofactor spectrum, notably increasing the absorbance at 340 nm, and demonstrated an interaction of the cofactor with the inhibitor sulfite. A crystal structure of LarA provided greater resolution (2.4 Å) than previously reported and revealed sulfite binding to the pyridinium C4 atom of the reduced pincer cofactor, mimicking hydride reduction during a PCHT catalytic cycle. Finally, computational modeling supports hydride transfer to the cofactor at the C4 position or to the nickel atom, but with formation of a nickel-hydride species requiring dissociation of the His200 metal ligand. In aggregate, these studies provide compelling evidence that the nickel-pincer cofactor acts by a PCHT mechanism.
中文翻译:

质子偶联的氢化物转移机制可操作乳酸消旋酶镍-Piner辅助因子。
植物乳杆菌的乳酸消旋酶(LarA)含有新颖的有机金属辅因子,其镍与共价键合的钳位配体吡啶-3-硫代酰胺-5-硫代羧酸单核苷酸配位,但尚未阐明其在酶机理中的作用。这项研究提供了直接的证据,表明镍-钳子辅助因子在LarA催化的乳酸外消旋作用中促进了质子偶联的氢化物转移(PCHT)机制。在不存在或存在底物的情况下,通过电子顺磁共振波谱未检测到LarA信号,这与+2金属氧化态一致且与先前提出的质子耦合电子转移机理不一致。在LarA的淬灭溶液中观察到丙酮酸(一种预测的PCHT机制中间体)。正常的底物动力学同位素效应(k H/ k D为3.11±0.17)使用2-α- 2确定H-乳酸,进一步支持PCHT机制。紫外可见光谱显示了乳酸引起的辅因子谱的扰动,特别是增加了在340 nm处的吸光度,并证明了辅因子与亚硫酸盐抑制剂的相互作用。LarA的晶体结构提供了比以前报道的更高的分辨率(2.4Å),并且揭示了亚硫酸盐与还原的钳夹辅助因子的吡啶鎓C4原子的结合,模仿了PCHT催化循环中氢化物的还原。最后,计算模型支持氢化物转移到C4位置的辅因子或镍原子,但形成氢化镍物质,需要解离His200金属配体。总体而言,这些研究提供了令人信服的证据,表明镍-钳子辅助因子通过PCHT机制起作用。
更新日期:2018-02-28
中文翻译:

质子偶联的氢化物转移机制可操作乳酸消旋酶镍-Piner辅助因子。
植物乳杆菌的乳酸消旋酶(LarA)含有新颖的有机金属辅因子,其镍与共价键合的钳位配体吡啶-3-硫代酰胺-5-硫代羧酸单核苷酸配位,但尚未阐明其在酶机理中的作用。这项研究提供了直接的证据,表明镍-钳子辅助因子在LarA催化的乳酸外消旋作用中促进了质子偶联的氢化物转移(PCHT)机制。在不存在或存在底物的情况下,通过电子顺磁共振波谱未检测到LarA信号,这与+2金属氧化态一致且与先前提出的质子耦合电子转移机理不一致。在LarA的淬灭溶液中观察到丙酮酸(一种预测的PCHT机制中间体)。正常的底物动力学同位素效应(k H/ k D为3.11±0.17)使用2-α- 2确定H-乳酸,进一步支持PCHT机制。紫外可见光谱显示了乳酸引起的辅因子谱的扰动,特别是增加了在340 nm处的吸光度,并证明了辅因子与亚硫酸盐抑制剂的相互作用。LarA的晶体结构提供了比以前报道的更高的分辨率(2.4Å),并且揭示了亚硫酸盐与还原的钳夹辅助因子的吡啶鎓C4原子的结合,模仿了PCHT催化循环中氢化物的还原。最后,计算模型支持氢化物转移到C4位置的辅因子或镍原子,但形成氢化镍物质,需要解离His200金属配体。总体而言,这些研究提供了令人信服的证据,表明镍-钳子辅助因子通过PCHT机制起作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号