当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, Structure–Activity Relationship Studies, and ADMET Properties of 3‐Aminocyclohex‐2‐en‐1‐ones as Chemokine Receptor 2 (CXCR2) Antagonists
ChemMedChem ( IF 3.6 ) Pub Date : 2018-04-30 , DOI: 10.1002/cmdc.201800027 Weiyang Dai 1, 2 , Wenmin Chen 1 , Bikash Debnath 1 , Yong Wu 2 , Nouri Neamati 1
ChemMedChem ( IF 3.6 ) Pub Date : 2018-04-30 , DOI: 10.1002/cmdc.201800027 Weiyang Dai 1, 2 , Wenmin Chen 1 , Bikash Debnath 1 , Yong Wu 2 , Nouri Neamati 1
Affiliation
|
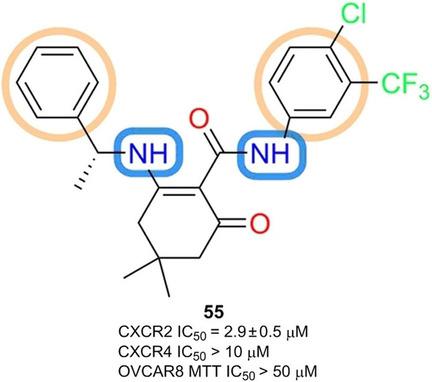
Herein we describe the synthesis and structure–activity relationships of 3‐aminocyclohex‐2‐en‐1‐one derivatives as novel chemokine receptor 2 (CXCR2) antagonists. Thirteen out of 44 derivatives were found to inhibit CXCR2 downstream signaling in a Tango assay specific for CXCR2, with IC50 values less than 10 μm. In silico ADMET prediction suggests that all active compounds possess drug‐like properties. None of these compounds show significant cytotoxicity, suggesting their potential application in inflammatory mediated diseases. A structure–activity relationship (SAR) map has been generated to gain better understanding of their binding mechanism to guide further optimization of these new CXCR2 antagonists.
中文翻译:

合成,结构-活性关系研究和作为趋化因子受体2(CXCR2)拮抗剂的3-氨基环己-2-烯-1-酮的ADMET性质
在这里,我们描述了作为新型趋化因子受体2(CXCR2)拮抗剂的3-氨基环己-2-烯-1-酮衍生物的合成及其与构效关系。在针对CXCR2的探戈分析中,发现44种衍生物中的13种抑制CXCR2下游信号传导,IC 50值小于10μm。在计算机模拟中ADMET的预测表明,所有活性化合物都具有类似药物的特性。这些化合物均未显示出明显的细胞毒性,表明它们在炎症介导的疾病中的潜在应用。已经生成了结构-活性关系(SAR)图,以更好地了解它们的结合机制,以指导进一步优化这些新的CXCR2拮抗剂。
更新日期:2018-04-30
中文翻译:

合成,结构-活性关系研究和作为趋化因子受体2(CXCR2)拮抗剂的3-氨基环己-2-烯-1-酮的ADMET性质
在这里,我们描述了作为新型趋化因子受体2(CXCR2)拮抗剂的3-氨基环己-2-烯-1-酮衍生物的合成及其与构效关系。在针对CXCR2的探戈分析中,发现44种衍生物中的13种抑制CXCR2下游信号传导,IC 50值小于10μm。在计算机模拟中ADMET的预测表明,所有活性化合物都具有类似药物的特性。这些化合物均未显示出明显的细胞毒性,表明它们在炎症介导的疾病中的潜在应用。已经生成了结构-活性关系(SAR)图,以更好地了解它们的结合机制,以指导进一步优化这些新的CXCR2拮抗剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号