当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Organometallic halogen bond acceptors: directionality, hybrid cocrystal precipitation, and blueshifted CO ligand vibrational band†
CrystEngComm ( IF 3.1 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1039/c7ce02185b Yury V. Torubaev 1, 2, 3, 4 , Ivan V. Skabitskiy 1, 2, 3, 4 , Polina Rusina 4, 5, 6 , Alexander A. Pasynskii 1, 2, 3, 4 , Dhirendra K. Rai 7, 8, 9, 10 , Ajeet Singh 8, 9, 10, 11
CrystEngComm ( IF 3.1 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1039/c7ce02185b Yury V. Torubaev 1, 2, 3, 4 , Ivan V. Skabitskiy 1, 2, 3, 4 , Polina Rusina 4, 5, 6 , Alexander A. Pasynskii 1, 2, 3, 4 , Dhirendra K. Rai 7, 8, 9, 10 , Ajeet Singh 8, 9, 10, 11
Affiliation
|
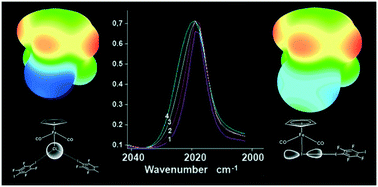
Iron cyclopentadienyl carbonyl-halide and -chalcogenolate complexes CpFe(CO)2X (X = Cl, Br, I, TePh, SPh) readily afford cocrystals with the bidentate halogen bond donor 1,4-diiodotetrafluorobenzene (p-DITFB) under slow evaporation or vapor diffusion conditions. The same microcrystalline [CpFe(CO)2TePh](p-DITFB) product instantly precipitates upon mixing p-DITFB and CpFe(CO)2TePh in hexane solution. Supramolecular [CpFe(CO)2X(p-DITFB)]n chains in the cocrystals are assembled by halogen bonds (XB) between the electrophilic area of iodine atoms of p-DITFB and the nucleophilic area of X in CpFe(CO)nX. The 5–10 cm−1 hypsochromic shift of the CO stretching bands in the IR spectra of [CpFe(CO)2X(p-DITFB)] cocrystals is explained by the pronounced electron-withdrawing effect of halogen bonding (XB), as supported by DFT calculations. The observed influence of the nature of the XB acceptor (X) on the XB geometry is described in terms of hybridization and electrostatic surface potential (ESP) mapping.
中文翻译:

有机金属卤素键受体:方向性,混合共晶体沉淀和蓝移的CO配体振动带†
环戊二烯基羰基卤化铁和硫属元素化物络合物CpFe(CO)2 X(X = Cl,Br,I,TePh,SPh)在缓慢蒸发的情况下容易与双齿卤素键供体1,4-二碘四氟苯(p -DITFB)形成共晶体或蒸汽扩散条件。在己烷溶液中将p -DITFB和CpFe(CO)2 TePh混合后,相同的微晶[CpFe(CO)2 TePh](p -DITFB)产物立即沉淀。共晶中的超分子[CpFe(CO)2 X(p -DITFB)] n链通过p碘原子亲电区之间的卤素键(XB)组装-DITFB和CpFe(CO)n X中X的亲核区域。在[CpFe(CO)2 X(p -DITFB)]共晶体的红外光谱中,CO拉伸带的5–10 cm -1变色位移为由DFT计算所支持的卤素键(XB)的显着吸电子效应解释了这一点。XB受体(X)的性质对XB几何形状的观察影响通过杂交和静电表面电势(ESP)映射进行描述。
更新日期:2018-02-28
中文翻译:

有机金属卤素键受体:方向性,混合共晶体沉淀和蓝移的CO配体振动带†
环戊二烯基羰基卤化铁和硫属元素化物络合物CpFe(CO)2 X(X = Cl,Br,I,TePh,SPh)在缓慢蒸发的情况下容易与双齿卤素键供体1,4-二碘四氟苯(p -DITFB)形成共晶体或蒸汽扩散条件。在己烷溶液中将p -DITFB和CpFe(CO)2 TePh混合后,相同的微晶[CpFe(CO)2 TePh](p -DITFB)产物立即沉淀。共晶中的超分子[CpFe(CO)2 X(p -DITFB)] n链通过p碘原子亲电区之间的卤素键(XB)组装-DITFB和CpFe(CO)n X中X的亲核区域。在[CpFe(CO)2 X(p -DITFB)]共晶体的红外光谱中,CO拉伸带的5–10 cm -1变色位移为由DFT计算所支持的卤素键(XB)的显着吸电子效应解释了这一点。XB受体(X)的性质对XB几何形状的观察影响通过杂交和静电表面电势(ESP)映射进行描述。



























 京公网安备 11010802027423号
京公网安备 11010802027423号