当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modular peptide-functionalized gold nanorods for effective glioblastoma multicellular tumor spheroid targeting†
Biomaterials Science ( IF 6.6 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1039/c7bm01107e D. P. N. Gonçalves 1, 2, 3 , D. M. Park 4, 5, 6, 7, 8 , T. L. Schmidt 3, 9, 10, 11 , C. Werner 1, 2, 3
Biomaterials Science ( IF 6.6 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1039/c7bm01107e D. P. N. Gonçalves 1, 2, 3 , D. M. Park 4, 5, 6, 7, 8 , T. L. Schmidt 3, 9, 10, 11 , C. Werner 1, 2, 3
Affiliation
|
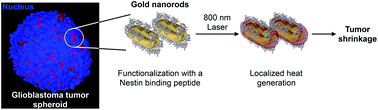
Glioblastoma multiforme (GBM) contains a population of tumor initiating stem-like cells, termed cancer stem cells (CSCs). These CSCs, which are resistant to chemo- and radiotherapy, are thought to persist after treatment and drive tumor recurrence. Thus, it is believed that the elimination of CSCs can lead to GBM remission. GBM CSCs express Nestin on their surface, and can be therefore targeted via this protein. Gold nanorods (AuNRs) functionalized with an engineered, modular peptide that recognizes Nestin (NesPEG-AuNRs) were used to target the models of solid tumors originated from human GBM CSC multicellular tumor spheroids (MCTS). In our study, we show that NesPEG-AuNRs have low cytotoxicity, are efficiently taken up by MCTS, and distribute uniformly throughout our tumor models, not only at the periphery as often seen in other nanoparticle systems. NesPEG-AuNR uptake by MCTS appears to be mediated by an energy/caveolae endocytic mechanism. Moreover, plasmon excitation of AuNRs in the near-infrared (NIR) region results in the production of localized heat. Consequently, NesPEG-AuNR cytotoxicity is only observed during NIR-irradiation in MCTS with a high intracellular AuNR content. The intracellular accumulation/diffusion of NesPEG-AuNRs and NIR-irradiation result in photothermally induced GBM CSC apoptosis and MCTS growth inhibition. In summary, these data suggest that the combination of the Nestin recognizing peptide with AuNRs contributes to better tumor accumulation/penetration, and thus in GBM CSC elimination. Moreover, due to the modularity of our peptide design, the Nestin-binding peptide sequence can be exchanged for peptides targeting other surface markers for the treatment of various types of tumors.
中文翻译:

模块化肽功能化金纳米棒可有效靶向胶质母细胞瘤多细胞肿瘤球体†
多形胶质母细胞瘤(GBM)包含一群肿瘤起始干细胞样细胞,称为癌症干细胞(CSC)。这些对化学疗法和放射疗法有抵抗力的CSC被认为在治疗后仍然存在,并促进了肿瘤的复发。因此,据信消除CSC可以导致GBM的减轻。GBM CSC在其表面表达Nestin,因此可以通过这种蛋白质。金纳米棒(AuNRs)用识别巢蛋白(NesPEG-AuNRs)的工程化,模块化肽功能化,用于靶向源自人GBM CSC多细胞肿瘤球体(MCTS)的实体瘤模型。在我们的研究中,我们显示NesPEG-AuNRs具有低细胞毒性,可被MCTS有效吸收,并均匀分布在我们的整个肿瘤模型中,不仅在其他纳米颗粒系统中经常出现在周围。MCTS对NesPEG-AuNR的吸收似乎是由能量/小泡内吞机制介导的。此外,近红外(NIR)区域中AuNRs的等离子体激元激发导致局部热量的产生。因此,仅在具有高细胞内AuNR含量的MCTS中在NIR辐照期间才观察到NesPEG-AuNR细胞毒性。NesPEG-AuNRs的细胞内积累/扩散和近红外辐射导致光热诱导的GBM CSC细胞凋亡和MCTS生长抑制。总之,这些数据表明,巢蛋白识别肽与AuNRs的组合有助于更好的肿瘤积累/穿透,从而有助于消除GBM CSC。此外,由于我们肽设计的模块化,可将Nestin结合肽序列交换为靶向其他表面标记物的肽,以治疗各种类型的肿瘤。
更新日期:2018-02-28
中文翻译:

模块化肽功能化金纳米棒可有效靶向胶质母细胞瘤多细胞肿瘤球体†
多形胶质母细胞瘤(GBM)包含一群肿瘤起始干细胞样细胞,称为癌症干细胞(CSC)。这些对化学疗法和放射疗法有抵抗力的CSC被认为在治疗后仍然存在,并促进了肿瘤的复发。因此,据信消除CSC可以导致GBM的减轻。GBM CSC在其表面表达Nestin,因此可以通过这种蛋白质。金纳米棒(AuNRs)用识别巢蛋白(NesPEG-AuNRs)的工程化,模块化肽功能化,用于靶向源自人GBM CSC多细胞肿瘤球体(MCTS)的实体瘤模型。在我们的研究中,我们显示NesPEG-AuNRs具有低细胞毒性,可被MCTS有效吸收,并均匀分布在我们的整个肿瘤模型中,不仅在其他纳米颗粒系统中经常出现在周围。MCTS对NesPEG-AuNR的吸收似乎是由能量/小泡内吞机制介导的。此外,近红外(NIR)区域中AuNRs的等离子体激元激发导致局部热量的产生。因此,仅在具有高细胞内AuNR含量的MCTS中在NIR辐照期间才观察到NesPEG-AuNR细胞毒性。NesPEG-AuNRs的细胞内积累/扩散和近红外辐射导致光热诱导的GBM CSC细胞凋亡和MCTS生长抑制。总之,这些数据表明,巢蛋白识别肽与AuNRs的组合有助于更好的肿瘤积累/穿透,从而有助于消除GBM CSC。此外,由于我们肽设计的模块化,可将Nestin结合肽序列交换为靶向其他表面标记物的肽,以治疗各种类型的肿瘤。



























 京公网安备 11010802027423号
京公网安备 11010802027423号