当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How Methylation Modifies the Photophysics of the Native All-trans-Retinal Protonated Schiff Base: A CASPT2/MD Study in Gas Phase and in Methanol
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1021/acs.jpca.8b00773 Rute Barata-Morgado 1 , M. Luz Sánchez 1 , Aurora Muñoz-Losa 2 , M. Elena Martín 1 , Francisco J. Olivares del Valle 1 , Manuel A. Aguilar 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1021/acs.jpca.8b00773 Rute Barata-Morgado 1 , M. Luz Sánchez 1 , Aurora Muñoz-Losa 2 , M. Elena Martín 1 , Francisco J. Olivares del Valle 1 , Manuel A. Aguilar 1
Affiliation
|
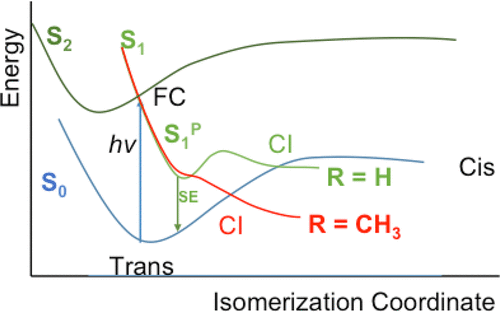
A comparison between the free-energy surfaces of the all-trans-retinal protonated Schiff base (RPSB) and its 10-methylated derivative in gas phase and methanol solution is performed at CASSCF//CASSCF and CASPT2//CASSCF levels. Solvent effects were included using the average solvent electrostatic potential from molecular dynamics method. This is a QM/MM (quantum mechanics/molecular mechanics) method that makes use of the mean field approximation. It is found that the methyl group bonded to C10 produces noticeable changes in the solution free-energy profile of the S1 excited state, mainly in the relative stability of the minimum energy conical intersections (MECIs) with respect to the Franck–Condon (FC) point. The conical intersections yielding the 9-cis and 11-cis isomers are stabilized while that yielding the 13-cis isomer is destabilized; in fact, it becomes inaccessible by excitation to S1. Furthermore, the planar S1 minimum is not present in the methylated compound. The solvent notably stabilizes the S2 excited state at the FC geometry. Therefore, if the S2 state has an effect on the photoisomerization dynamics, it must be because it permits the RPSB population to branch around the FC point. All these changes combine to speed up the photoisomerization in the 10-methylated compound with respect to the native compound.
中文翻译:

甲基化如何修饰天然全反式视网膜质子化席夫碱的光物理性质:气相和甲醇中的CASPT2 / MD研究
在CASSCF // CASSCF和CASPT2 // CASSCF水平下,对全反式视网膜质子化席夫碱(RPSB)及其10-甲基化衍生物在气相和甲醇溶液中的自由能表面进行了比较。使用来自分子动力学方法的平均溶剂静电势包括溶剂效果。这是利用平均场近似的QM / MM(量子力学/分子力学)方法。发现与C10键合的甲基在S 1激发态的溶液自由能分布中产生了显着变化,主要是相对于Franck-Condon(FC)的最小能量圆锥形交点(MECI)的相对稳定性) 观点。圆锥形相交产生9-顺式和11-顺式异构体是稳定的,而产生13-顺式异构体的是不稳定的;实际上,通过激励S 1变得不可访问。此外,在甲基化化合物中不存在平面S 1最小值。溶剂在FC几何形状上特别稳定了S 2激发态。因此,如果S 2状态对光异构化动力学有影响,那一定是因为它允许RPSB群体在FC点附近分支。所有这些变化相结合,以加速10-甲基化化合物相对于天然化合物的光异构化。
更新日期:2018-02-28
中文翻译:

甲基化如何修饰天然全反式视网膜质子化席夫碱的光物理性质:气相和甲醇中的CASPT2 / MD研究
在CASSCF // CASSCF和CASPT2 // CASSCF水平下,对全反式视网膜质子化席夫碱(RPSB)及其10-甲基化衍生物在气相和甲醇溶液中的自由能表面进行了比较。使用来自分子动力学方法的平均溶剂静电势包括溶剂效果。这是利用平均场近似的QM / MM(量子力学/分子力学)方法。发现与C10键合的甲基在S 1激发态的溶液自由能分布中产生了显着变化,主要是相对于Franck-Condon(FC)的最小能量圆锥形交点(MECI)的相对稳定性) 观点。圆锥形相交产生9-顺式和11-顺式异构体是稳定的,而产生13-顺式异构体的是不稳定的;实际上,通过激励S 1变得不可访问。此外,在甲基化化合物中不存在平面S 1最小值。溶剂在FC几何形状上特别稳定了S 2激发态。因此,如果S 2状态对光异构化动力学有影响,那一定是因为它允许RPSB群体在FC点附近分支。所有这些变化相结合,以加速10-甲基化化合物相对于天然化合物的光异构化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号