当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lewis-Brønsted acid pairs in Ga/H-ZSM-5 to catalyze dehydrogenation of light alkanes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-02-28 , DOI: 10.1021/jacs.7b12901 Moritz W. Schreiber 1 , Craig P. Plaisance 1 , Martin Baumgärtl 1 , Karsten Reuter 1 , Andreas Jentys 1 , Ricardo Bermejo-Deval 1 , Johannes A. Lercher 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-02-28 , DOI: 10.1021/jacs.7b12901 Moritz W. Schreiber 1 , Craig P. Plaisance 1 , Martin Baumgärtl 1 , Karsten Reuter 1 , Andreas Jentys 1 , Ricardo Bermejo-Deval 1 , Johannes A. Lercher 1
Affiliation
|
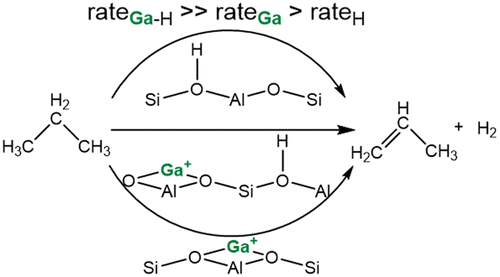
The active sites for propane dehydrogenation in Ga/H-ZSM-5 with moderate concentrations of tetrahedral aluminum in the lattice were identified to be Lewis-Brønsted acid pairs. With increasing availability, Ga+ and Brønsted acid site concentrations changed inversely, as protons of Brønsted acid sites were exchanged with Ga+. At a Ga/Al ratio of 1/2, the rate of propane dehydrogenation was 2 orders of magnitude higher than with the parent H-ZSM-5, highlighting the extraordinary activity of the Lewis-Brønsted acid pairs. Density functional theory calculations relate the high activity to a bifunctional mechanism that proceeds via heterolytic activation of the propane C-H bond followed by a monomolecular elimination of H2 and desorption of propene.
中文翻译:

Ga/H-ZSM-5中的Lewis-Brønsted酸对催化轻烷烃脱氢
Ga/H-ZSM-5 中丙烷脱氢的活性位点在晶格中具有中等浓度的四面体铝,被确定为路易斯-布朗斯台德酸对。随着可用性的增加,Ga+ 和 Brønsted 酸位点浓度发生相反的变化,因为 Brønsted 酸位点的质子与 Ga+ 交换。在 Ga/Al 比为 1/2 时,丙烷脱氢速率比母体 H-ZSM-5 高 2 个数量级,突出了 Lewis-Brønsted 酸对的非凡活性。密度泛函理论计算将高活性与双功能机制联系起来,该机制通过丙烷 CH 键的异裂活化进行,然后是 H2 的单分子消除和丙烯的解吸。
更新日期:2018-02-28
中文翻译:

Ga/H-ZSM-5中的Lewis-Brønsted酸对催化轻烷烃脱氢
Ga/H-ZSM-5 中丙烷脱氢的活性位点在晶格中具有中等浓度的四面体铝,被确定为路易斯-布朗斯台德酸对。随着可用性的增加,Ga+ 和 Brønsted 酸位点浓度发生相反的变化,因为 Brønsted 酸位点的质子与 Ga+ 交换。在 Ga/Al 比为 1/2 时,丙烷脱氢速率比母体 H-ZSM-5 高 2 个数量级,突出了 Lewis-Brønsted 酸对的非凡活性。密度泛函理论计算将高活性与双功能机制联系起来,该机制通过丙烷 CH 键的异裂活化进行,然后是 H2 的单分子消除和丙烯的解吸。











































 京公网安备 11010802027423号
京公网安备 11010802027423号