当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ruthenium-catalyzed dynamic kinetic asymmetric transfer hydrogenation: stereoselective access to syn 2-(1,2,3,4-tetrahydro-1-isoquinolyl)ethanol derivatives†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1039/c7qo01142c Long-Sheng Zheng 1, 2, 3, 4, 5 , Phannarath Phansavath 1, 2, 3, 4, 5 , Virginie Ratovelomanana-Vidal 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1039/c7qo01142c Long-Sheng Zheng 1, 2, 3, 4, 5 , Phannarath Phansavath 1, 2, 3, 4, 5 , Virginie Ratovelomanana-Vidal 1, 2, 3, 4, 5
Affiliation
|
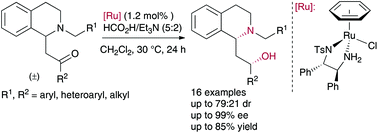
An efficient, stereoselective route to 2-(1,2,3,4-tetrahydro-1-isoquinolyl)ethanol derivatives through an asymmetric transfer hydrogenation of ring-substituted β-amino ketones via dynamic kinetic resolution has been developed. The reaction proceeded under mild conditions in the presence of RuCl[(S,S)-TsDPEN](benzene) and HCO2H/Et3N (5 : 2) as the hydrogen source, delivering in good yields a variety of syn 2-(1,2,3,4-tetrahydro-1-isoquinolyl)ethanol derivatives with excellent enantioselectivities (up to 99% ee) and diastereomeric ratios up to 71 : 29 dr.
中文翻译:

钌催化的动态动力学不对称转移氢化反应:立体选择性地获得合成的2-(1,2,3,4-四氢-1-异喹啉基)乙醇衍生物†
已经开发了通过动态动力学拆分通过环取代的β-氨基酮的不对称转移氢化而形成2-(1,2,3,4-四氢-1-异喹啉基)乙醇衍生物的有效的立体选择性途径。反应在温和的条件下,在RuCl [(S,S)-TsDPEN](苯)和HCO 2 H / Et 3 N(5:2)作为氢源的条件下进行,以高产率提供了多种syn 2 -(1,2,3,4-四氢-1-异喹啉基)乙醇衍生物,具有出色的对映选择性(高达99%ee)和非对映体比率高达71:29 dr。
更新日期:2018-02-28
中文翻译:

钌催化的动态动力学不对称转移氢化反应:立体选择性地获得合成的2-(1,2,3,4-四氢-1-异喹啉基)乙醇衍生物†
已经开发了通过动态动力学拆分通过环取代的β-氨基酮的不对称转移氢化而形成2-(1,2,3,4-四氢-1-异喹啉基)乙醇衍生物的有效的立体选择性途径。反应在温和的条件下,在RuCl [(S,S)-TsDPEN](苯)和HCO 2 H / Et 3 N(5:2)作为氢源的条件下进行,以高产率提供了多种syn 2 -(1,2,3,4-四氢-1-异喹啉基)乙醇衍生物,具有出色的对映选择性(高达99%ee)和非对映体比率高达71:29 dr。











































 京公网安备 11010802027423号
京公网安备 11010802027423号