当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Use of stability diagrams to predict catalyst speciation during Fischer Tropsch reduction stage: a mini-review
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-03-01 00:00:00 , DOI: 10.1039/c8cy00228b Joshua Gorimbo 1, 2, 3
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-03-01 00:00:00 , DOI: 10.1039/c8cy00228b Joshua Gorimbo 1, 2, 3
Affiliation
|
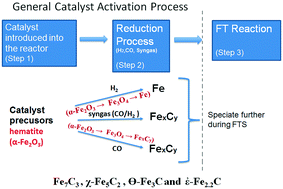
An understanding of the speciation of iron catalyst precursor during reduction is an important piece of knowledge in Fischer–Tropsch Synthesis (FTS) reaction. The thermodynamics of the precursor speciation is governed by the reducing gases used, and this tends to influence the activity, selectivity and the catalyst life span. During catalyst reduction or FT synthesis, the interaction of reducing agents with Fe-based catalyst results in the formation of several gaseous components such as CO, H2, CO2 and H2O. The partial pressure of each gaseous component and operating conditions determine the predominant state of the catalyst. Catalyst speciation happens due to different partial pressures of the gaseous components, and as a result, the stable iron phases formed during catalyst reduction are those that are in equilibrium with the gas composition. Although the ratio of PH2/PH2O and PCO/PCO2 or the partial pressure of water and carbon dioxide does not have any appreciable effect on the deactivation by oxidation on the FT reaction rate in Cobalt catalysts, in the iron based catalyst deactivation by oxidation is predominant. Based on thermodynamic calculations of the FTS system, the maximum allowable oxygen partial pressure during catalyst activation is 1 × 10−45 bars. The oxygen partial pressure is therefore dependent on the PH2/PH2O and PCO2/PCO ratios, and the equilibrium constants.
中文翻译:

使用稳定性图预测费-托还原阶段的催化剂形态:小型综述
还原过程中对铁催化剂前体形态的了解是费托合成(FTS)反应的重要知识。前体形态的热力学受所用还原气体的控制,这往往会影响活性,选择性和催化剂的寿命。在催化剂还原或FT合成过程中,还原剂与铁基催化剂的相互作用导致形成几种气态组分,例如CO,H 2,CO 2和H 2O.每种气态组分的分压和操作条件决定了催化剂的主要状态。催化剂形成是由于气态组分的分压不同而引起的,因此,在催化剂还原过程中形成的稳定铁相就是那些与气体组成平衡的铁相。虽然P H2 / P H2O与P CO / P CO2之比或水和二氧化碳的分压对钴催化剂中FT反应速率的氧化反应失活没有明显影响,其中铁基催化剂的氧化失活是主要的。根据FTS系统的热力学计算,催化剂活化过程中的最大允许氧分压为1×10 -45 bar。因此,氧分压取决于P H 2 / P H 2 O和P CO 2 / P CO的比例以及平衡常数。
更新日期:2018-03-01
中文翻译:

使用稳定性图预测费-托还原阶段的催化剂形态:小型综述
还原过程中对铁催化剂前体形态的了解是费托合成(FTS)反应的重要知识。前体形态的热力学受所用还原气体的控制,这往往会影响活性,选择性和催化剂的寿命。在催化剂还原或FT合成过程中,还原剂与铁基催化剂的相互作用导致形成几种气态组分,例如CO,H 2,CO 2和H 2O.每种气态组分的分压和操作条件决定了催化剂的主要状态。催化剂形成是由于气态组分的分压不同而引起的,因此,在催化剂还原过程中形成的稳定铁相就是那些与气体组成平衡的铁相。虽然P H2 / P H2O与P CO / P CO2之比或水和二氧化碳的分压对钴催化剂中FT反应速率的氧化反应失活没有明显影响,其中铁基催化剂的氧化失活是主要的。根据FTS系统的热力学计算,催化剂活化过程中的最大允许氧分压为1×10 -45 bar。因此,氧分压取决于P H 2 / P H 2 O和P CO 2 / P CO的比例以及平衡常数。











































 京公网安备 11010802027423号
京公网安备 11010802027423号