当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New activation mechanism for half-sandwich organometallic anticancer complexes†
Chemical Science ( IF 7.6 ) Pub Date : 2018-03-01 00:00:00 , DOI: 10.1039/c7sc05058e Samya Banerjee 1 , Joan J Soldevila-Barreda 1 , Juliusz A Wolny 2 , Christopher A Wootton 1 , Abraha Habtemariam 1 , Isolda Romero-Canelón 1 , Feng Chen 1 , Guy J Clarkson 1 , Ivan Prokes 1 , Lijiang Song 1 , Peter B O'Connor 1 , Volker Schünemann 2 , Peter J Sadler 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-03-01 00:00:00 , DOI: 10.1039/c7sc05058e Samya Banerjee 1 , Joan J Soldevila-Barreda 1 , Juliusz A Wolny 2 , Christopher A Wootton 1 , Abraha Habtemariam 1 , Isolda Romero-Canelón 1 , Feng Chen 1 , Guy J Clarkson 1 , Ivan Prokes 1 , Lijiang Song 1 , Peter B O'Connor 1 , Volker Schünemann 2 , Peter J Sadler 1
Affiliation
|
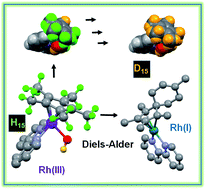
The Cpx C–H protons in certain organometallic RhIII half-sandwich anticancer complexes [(η5-Cpx)Rh(N,N′)Cl]+, where Cpx = Cp*, phenyl or biphenyl-Me4Cp, and N,N′ = bipyridine, dimethylbipyridine, or phenanthroline, can undergo rapid sequential deuteration of all 15 Cp* methyl protons in aqueous media at ambient temperature. DFT calculations suggest a mechanism involving abstraction of a Cp* proton by the Rh–hydroxido complex, followed by sequential H/D exchange, with the Cp* rings behaving like dynamic molecular ‘twisters’. The calculations reveal the crucial role of pπ orbitals of N,N′-chelated ligands in stabilizing deprotonated Cpx ligands, and also the accessibility of RhI–fulvene intermediates. They also provide insight into why biologically-inactive complexes such as [(Cp*)RhIII(en)Cl]+ and [(Cp*)IrIII(bpy)Cl]+ do not have activated Cp* rings. The thiol tripeptide glutathione (γ-L-Glu-L-Cys-Gly, GSH) and the activated dienophile N-methylmaleimide, (NMM) did not undergo addition reactions with the proposed RhI–fulvene, although they were able to control the extent of Cp* deuteration. We readily trapped and characterized RhI–fulvene intermediates by Diels–Alder [4+2] cyclo-addition reactions with the natural biological dienes isoprene and conjugated (9Z,11E)-linoleic acid in aqueous media, including cell culture medium, the first report of a Diels–Alder reaction of a metal-bound fulvene in aqueous solution. These findings will introduce new concepts into the design of organometallic Cp* anticancer complexes with novel mechanisms of action.
中文翻译:

半夹心有机金属抗癌复合物的新活化机制†
某些有机金属 Rh III半夹心抗癌配合物 [(η 5 -Cp x )Rh( N , N ')Cl] +中的 Cp x C–H 质子,其中 Cp x = Cp*,苯基或联苯-Me 4 Cp , 和N , N' = 联吡啶、二甲基联吡啶或菲咯啉,可以在环境温度下对水介质中的所有 15 Cp* 甲基质子进行快速连续氘化。DFT 计算表明了一种机制,涉及通过 Rh-羟基络合物提取 Cp* 质子,然后进行顺序 H/D 交换,其中 Cp* 环表现得像动态分子“扭曲器”。计算揭示了N , N '-螯合配体的 p π轨道在稳定去质子化 Cp x配体中的关键作用,以及 Rh I -富烯中间体的可及性。它们还提供了对为何不具有生物活性的复合物如 [(Cp*)Rh III (en)Cl] +和 [(Cp*)IrIII (bpy)Cl] +没有活化的 Cp* 环。硫醇三肽谷胱苷_ _ _ Cp* 氘化程度。我们通过 Diels-Alder [4+2] 环加成反应与天然生物二烯异戊二烯和共轭 (9 Z , 11 E)-亚油酸在水性介质(包括细胞培养基)中,首次报道了金属结合富烯在水溶液中的 Diels-Alder 反应。这些发现将为具有新作用机制的有机金属 Cp* 抗癌复合物的设计引入新概念。
更新日期:2018-03-01
中文翻译:

半夹心有机金属抗癌复合物的新活化机制†
某些有机金属 Rh III半夹心抗癌配合物 [(η 5 -Cp x )Rh( N , N ')Cl] +中的 Cp x C–H 质子,其中 Cp x = Cp*,苯基或联苯-Me 4 Cp , 和N , N' = 联吡啶、二甲基联吡啶或菲咯啉,可以在环境温度下对水介质中的所有 15 Cp* 甲基质子进行快速连续氘化。DFT 计算表明了一种机制,涉及通过 Rh-羟基络合物提取 Cp* 质子,然后进行顺序 H/D 交换,其中 Cp* 环表现得像动态分子“扭曲器”。计算揭示了N , N '-螯合配体的 p π轨道在稳定去质子化 Cp x配体中的关键作用,以及 Rh I -富烯中间体的可及性。它们还提供了对为何不具有生物活性的复合物如 [(Cp*)Rh III (en)Cl] +和 [(Cp*)IrIII (bpy)Cl] +没有活化的 Cp* 环。硫醇三肽谷胱苷_ _ _ Cp* 氘化程度。我们通过 Diels-Alder [4+2] 环加成反应与天然生物二烯异戊二烯和共轭 (9 Z , 11 E)-亚油酸在水性介质(包括细胞培养基)中,首次报道了金属结合富烯在水溶液中的 Diels-Alder 反应。这些发现将为具有新作用机制的有机金属 Cp* 抗癌复合物的设计引入新概念。











































 京公网安备 11010802027423号
京公网安备 11010802027423号