当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Taking Advantage of Hydrophobic Fluorine Interactions for Self‐Assembled Quantum Dots as a Delivery Platform for Enzymes
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-03-23 , DOI: 10.1002/anie.201801155 Carolina Carrillo-Carrion 1 , Mona Atabakhshi-Kashi 2 , Mónica Carril 1, 3 , Khosro Khajeh 2 , Wolfgang J. Parak 1, 4
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-03-23 , DOI: 10.1002/anie.201801155 Carolina Carrillo-Carrion 1 , Mona Atabakhshi-Kashi 2 , Mónica Carril 1, 3 , Khosro Khajeh 2 , Wolfgang J. Parak 1, 4
Affiliation
|
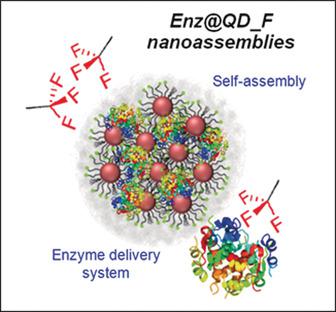
Self‐assembly of nanoparticles provides unique opportunities as nanoplatforms for controlled delivery. By exploiting the important role of noncovalent hydrophobic interactions in the engineering of stable assemblies, nanoassemblies were formed by the self‐assembly of fluorinated quantum dots in aqueous medium through fluorine–fluorine interactions. These nanoassemblies encapsulated different enzymes (laccase and α‐galactosidase) with encapsulation efficiencies of ≥74 %. Importantly, the encapsulated enzymes maintained their catalytic activity, following Michaelis–Menten kinetics. Under an acidic environment the nanoassemblies were slowly disassembled, thus allowing the release of encapsulated enzymes. The effective release of the assayed enzymes demonstrated the feasibility of this nanoplatform to be used in pH‐mediated enzyme delivery. In addition, the as‐synthesized nanoassemblies, having a diameter of about 50 nm, presented high colloidal stability and fluorescence emission, which make them a promising multifunctional nanoplatform.
中文翻译:

利用疏水性氟相互作用自组装量子点作为酶的传递平台
纳米粒子的自组装提供了独特的机会,可控制交付的纳米平台。通过利用非共价疏水相互作用在稳定组装工程中的重要作用,纳米组装件是通过氟-氟相互作用在水性介质中自组装氟化量子点而形成的。这些纳米组件封装了不同的酶(漆酶和α-半乳糖苷酶),封装效率≥74%。重要的是,遵循米利斯-门腾动力学,封装的酶保持了其催化活性。在酸性环境下,纳米组件会缓慢分解,从而释放出包封的酶。被测定酶的有效释放证明了该纳米平台在pH介导的酶递送中的可行性。
更新日期:2018-03-23
中文翻译:

利用疏水性氟相互作用自组装量子点作为酶的传递平台
纳米粒子的自组装提供了独特的机会,可控制交付的纳米平台。通过利用非共价疏水相互作用在稳定组装工程中的重要作用,纳米组装件是通过氟-氟相互作用在水性介质中自组装氟化量子点而形成的。这些纳米组件封装了不同的酶(漆酶和α-半乳糖苷酶),封装效率≥74%。重要的是,遵循米利斯-门腾动力学,封装的酶保持了其催化活性。在酸性环境下,纳米组件会缓慢分解,从而释放出包封的酶。被测定酶的有效释放证明了该纳米平台在pH介导的酶递送中的可行性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号