当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lipase-catalyzed synthesis of chiral poly(ester amide)s with an alternating sequence of hydroxy acid and l/d-aspartate units†
Polymer Chemistry ( IF 4.1 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1039/c7py01936j Yiru Liang 1, 2, 3, 4 , Yu Zhang 1, 2, 3, 4 , Yujing Hu 1, 2, 3, 4 , Bo Xia 4, 5, 6, 7 , Xianfu Lin 1, 2, 3, 4 , Qi Wu 1, 2, 3, 4
Polymer Chemistry ( IF 4.1 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1039/c7py01936j Yiru Liang 1, 2, 3, 4 , Yu Zhang 1, 2, 3, 4 , Yujing Hu 1, 2, 3, 4 , Bo Xia 4, 5, 6, 7 , Xianfu Lin 1, 2, 3, 4 , Qi Wu 1, 2, 3, 4
Affiliation
|
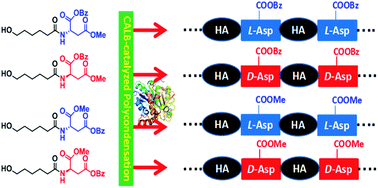
Most of reported synthesis approaches for poly(ester amide)s suffer from the difficulty in controlling the sequence of amino acid and ester units, and also some environmental disadvantages. Herein, we developed a facile approach for the lipase-catalyzed synthesis of chiral L-/D-Asp-based poly(ester amide)s with alternating aspartate and hydroxyhexanoic acid (HA) units. In the Novozym 435-catalyzed polycondensation starting from four monomers, namely N-(6-hydroxyhexanoyl) aspartate diesters with an L- or D-aspartate configuration, and methyl or benzyl ester groups at the α or β-carbonyl positions of Asp, we successfully obtained the corresponding alternating poly(HA-alt-L/D-β-aspartate)s with α-benzyl or α-methyl ester groups. The L-aspartate diester monomer was preferred to the D-aspartate diester monomer in the Novozym 435-catalyzed polycondensation, and it also showed high regioselectivity, mainly forming the β-linkage of the aspartate unit. Among the four poly(ester amide)s, poly(HA-alt-L-α-benzyl-β-aspartate) has the highest molar mass of up to 13 000 Da and 98% β-linkage. The thermal properties and the aggregate microstructures of these alternating poly(HA-alt-L/D-aspartate)s were also compared and some interesting differences were observed. Our results also showed that these enantiopure poly(HA-alt-L/D-aspartate)s did not form a stereocomplex.
中文翻译:

脂肪酶催化合成具有羟基酸和l / d-天冬氨酸单元交替序列的手性聚(酯酰胺)†
大多数报道的用于聚(酯酰胺)的合成方法都难以控制氨基酸和酯单元的序列,并且还具有一些环境不利因素。在这里,我们开发了一种简便的方法,用于脂肪酶催化的手性L- / D -Asp基聚(酯酰胺)与天冬氨酸和羟基己酸(HA)单元交替出现的合成。在Novozym 435催化的缩聚反应中,从四个单体开始,即具有L-或D-天冬氨酸构型的N-(6-羟基己酰基)天冬氨酸二酯,以及Asp的α或β-羰基位置的甲基或苄基酯基,成功地获得相应的交替的聚(HA- ALT -具有α-苄基或α-甲基酯基的L / D -β-天冬氨酸)。的大号天冬氨酸二酯单体优选将d天冬氨酸二酯单体在诺维信435催化的缩聚,并且它还表现出较高的区域选择性,主要形成天冬氨酸单元的β-联动。在这四种聚(酯酰胺)中,聚(HA - alt - L -α-苄基-β-天冬氨酸)的最高摩尔质量高达13000 Da,β-键合率为98%。这些交替的聚(HA- alt - L / D)的热学性质和聚集的微观结构-天冬氨酸)也进行了比较,观察到一些有趣的差异。我们的结果还表明,这些对映体纯的聚(HA- alt - L / D-天冬氨酸)不会形成立体复合物。
更新日期:2018-02-28
中文翻译:

脂肪酶催化合成具有羟基酸和l / d-天冬氨酸单元交替序列的手性聚(酯酰胺)†
大多数报道的用于聚(酯酰胺)的合成方法都难以控制氨基酸和酯单元的序列,并且还具有一些环境不利因素。在这里,我们开发了一种简便的方法,用于脂肪酶催化的手性L- / D -Asp基聚(酯酰胺)与天冬氨酸和羟基己酸(HA)单元交替出现的合成。在Novozym 435催化的缩聚反应中,从四个单体开始,即具有L-或D-天冬氨酸构型的N-(6-羟基己酰基)天冬氨酸二酯,以及Asp的α或β-羰基位置的甲基或苄基酯基,成功地获得相应的交替的聚(HA- ALT -具有α-苄基或α-甲基酯基的L / D -β-天冬氨酸)。的大号天冬氨酸二酯单体优选将d天冬氨酸二酯单体在诺维信435催化的缩聚,并且它还表现出较高的区域选择性,主要形成天冬氨酸单元的β-联动。在这四种聚(酯酰胺)中,聚(HA - alt - L -α-苄基-β-天冬氨酸)的最高摩尔质量高达13000 Da,β-键合率为98%。这些交替的聚(HA- alt - L / D)的热学性质和聚集的微观结构-天冬氨酸)也进行了比较,观察到一些有趣的差异。我们的结果还表明,这些对映体纯的聚(HA- alt - L / D-天冬氨酸)不会形成立体复合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号