当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal complexes of a novel heterocyclic benzimidazole ligand formed by rearrangement-cyclization of the corresponding Schiff base. Electrosynthesis, structural characterization and antimicrobial activity†
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1039/c8dt00532j I. Casanova 1, 2, 3, 4 , M. L. Durán 1, 2, 3, 4 , J. Viqueira 1, 2, 3, 4 , A. Sousa-Pedrares 1, 2, 3, 4 , F. Zani 5, 6, 7, 8 , J. A. Real 4, 9, 10, 11 , J. A. García-Vázquez 1, 2, 3, 4
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1039/c8dt00532j I. Casanova 1, 2, 3, 4 , M. L. Durán 1, 2, 3, 4 , J. Viqueira 1, 2, 3, 4 , A. Sousa-Pedrares 1, 2, 3, 4 , F. Zani 5, 6, 7, 8 , J. A. Real 4, 9, 10, 11 , J. A. García-Vázquez 1, 2, 3, 4
Affiliation
|
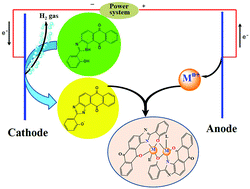
The electrochemical oxidation of anodic metals (M = cobalt, nickel, copper, zinc and cadmium) in a solution of the ligand 1H-anthra[1,2-d]imidazol-6,11-dione-2-[2-hydroxyphenyl] [H2L] afforded homoleptic [ML] compounds. The addition to the electrochemical cell of coligands (L′) such as 2,2′-bipyridine (bpy) or 1,10-phenanthroline (phen) allowed the synthesis, in one step, of heteroleptic [MLL′] compounds. The crystal structures of H2L (1), [CoL(MeOH)]2 (2), [CoL(phen)]2 (3), [NiL(bpy)]2 (4), [CuL(bpy)] (5), [CuL(phen)] (6) and [CdL(bpy)]2 (7) have been determined by X-ray diffraction techniques. The crystal structures of 2, 3, 4 and 7 consist of dimeric species in which both metallic atoms are connected through two phenolate bridges in a penta-coordinated (2) or hexa-coordinated (3, 4 and 7) environment. Copper compounds 5 and 6 are monomeric species with the metal in a pentacoordinated [N4O] environment. In all the compounds, the main interactions responsible for the crystal packing are classic (N–H⋯O, O–H⋯N and O–H⋯O) and non-classic (C–H⋯O and C–H⋯N) hydrogen bond interactions, and π interactions (π–π-stacking and C–H⋯π). All compounds were also characterized by microanalysis, IR spectroscopy, FAB mass spectrometry and 1H NMR spectroscopy. Magnetic susceptibility data were measured for 2–4 over the temperature range 2–300 K, and their analysis has revealed the occurrence of intramolecular antiferromagnetic coupling for 2 (J = −2 cm−1) and ferromagnetic coupling for 3 (J = 7.8 cm−1) and 4 (J = 2.8 cm−1) [J being the isotropic magnetic coupling parameter]. The nature of the magnetic coupling in 2–4 is correlated with the magnitude of the M–Ophenolate–M angle between the phenolate bridge and the metallic centers [M(II) = Co, Ni]. The in vitro antimicrobial properties of the novel ligand and its metal complexes were detected against Gram positive and Gram negative bacteria and fungi. [NiL(bpy)]2 and all tested Cd(II) complexes were the most active compounds, showing the highest inhibitory effect against bacilli (MIC 1.5–3 μg mL−1) and Sarcina, Streptococci and Haemophilus influenzae bacterial strains (MIC 12–50 μg mL−1), while almost no antifungal properties were observed.
中文翻译:

通过相应的席夫碱的重排-环化作用形成的新型杂环苯并咪唑配体的金属配合物。电合成,结构表征和抗菌活性†
配体1 H-蒽[1,2 - d ]咪唑-6,11-二酮-2- [2-羟基苯基]溶液中的阳极金属(M =钴,镍,铜,锌和镉)的电化学氧化[H 2 L]提供均匀的[ML]化合物。将大肠埃希氏菌(L')(例如2,2'-联吡啶(bpy)或1,10-菲咯啉(phen))添加到电化学电池中可以一步合成杂合[MLL']化合物。H 2 L(1),[CoL(MeOH)] 2(2),[CoL(phen)] 2(3),[NiL(bpy)] 2(4),[CuL(bpy)]的晶体结构(5),[CuL(phen)](6)和[CdL(bpy)] 2(7)已经通过X射线衍射技术确定。的晶体结构2,3,4和7组成,其中两个金属的原子以五配位(通过两个酚盐桥连接的二聚体种类的2)或六配位的(3,4和7)的环境。铜化合物5和6是单体物种,金属呈五配位[N 4O]环境。在所有化合物中,负责晶体堆积的主要相互作用是经典的(N–H⋯O,OH–H⋯N和OH–H⋯O)和非经典的(C–H⋯O和C–H⋯N )氢键相互作用和π相互作用(π–π堆积和C–H⋯π)。所有化合物还通过微分析,IR光谱,FAB质谱和1 H NMR光谱进行了表征。在2–300 K的温度范围内测量了2-4的磁化率数据,其分析表明发生了分子内反铁磁耦合2(J = -2 cm -1)和铁磁耦合3(J = 7.8 cm)的发生。-1)和4(J= 2.8cm -1)[J是各向同性的磁耦合参数]。2-4中磁耦合的性质与酚盐桥和金属中心之间的M–O酚盐–M角的大小相关[M(II)= Co,Ni]。在体外对革兰氏阳性和革兰氏阴性细菌和真菌进行检测的新型配体的抗微生物性质和它的金属络合物。[NiL(bpy)] 2和所有测试的Cd(II)配合物是活性最高的化合物,显示出对细菌(MIC 1.5–3μgmL -1)和Sarcina的抑制作用最高,链球菌和流感嗜血杆菌细菌菌株(MIC 12–50μgmL -1),而几乎未观察到抗真菌特性。
更新日期:2018-02-28
中文翻译:

通过相应的席夫碱的重排-环化作用形成的新型杂环苯并咪唑配体的金属配合物。电合成,结构表征和抗菌活性†
配体1 H-蒽[1,2 - d ]咪唑-6,11-二酮-2- [2-羟基苯基]溶液中的阳极金属(M =钴,镍,铜,锌和镉)的电化学氧化[H 2 L]提供均匀的[ML]化合物。将大肠埃希氏菌(L')(例如2,2'-联吡啶(bpy)或1,10-菲咯啉(phen))添加到电化学电池中可以一步合成杂合[MLL']化合物。H 2 L(1),[CoL(MeOH)] 2(2),[CoL(phen)] 2(3),[NiL(bpy)] 2(4),[CuL(bpy)]的晶体结构(5),[CuL(phen)](6)和[CdL(bpy)] 2(7)已经通过X射线衍射技术确定。的晶体结构2,3,4和7组成,其中两个金属的原子以五配位(通过两个酚盐桥连接的二聚体种类的2)或六配位的(3,4和7)的环境。铜化合物5和6是单体物种,金属呈五配位[N 4O]环境。在所有化合物中,负责晶体堆积的主要相互作用是经典的(N–H⋯O,OH–H⋯N和OH–H⋯O)和非经典的(C–H⋯O和C–H⋯N )氢键相互作用和π相互作用(π–π堆积和C–H⋯π)。所有化合物还通过微分析,IR光谱,FAB质谱和1 H NMR光谱进行了表征。在2–300 K的温度范围内测量了2-4的磁化率数据,其分析表明发生了分子内反铁磁耦合2(J = -2 cm -1)和铁磁耦合3(J = 7.8 cm)的发生。-1)和4(J= 2.8cm -1)[J是各向同性的磁耦合参数]。2-4中磁耦合的性质与酚盐桥和金属中心之间的M–O酚盐–M角的大小相关[M(II)= Co,Ni]。在体外对革兰氏阳性和革兰氏阴性细菌和真菌进行检测的新型配体的抗微生物性质和它的金属络合物。[NiL(bpy)] 2和所有测试的Cd(II)配合物是活性最高的化合物,显示出对细菌(MIC 1.5–3μgmL -1)和Sarcina的抑制作用最高,链球菌和流感嗜血杆菌细菌菌株(MIC 12–50μgmL -1),而几乎未观察到抗真菌特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号