当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Necessary and sufficient conditions for the successful three-phase photocatalytic reduction of CO2 by H2O over heterogeneous photocatalysts
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1039/c7cp07783a Kentaro Teramura 1, 2, 3, 4, 5 , Tsunehiro Tanaka 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1039/c7cp07783a Kentaro Teramura 1, 2, 3, 4, 5 , Tsunehiro Tanaka 1, 2, 3, 4, 5
Affiliation
|
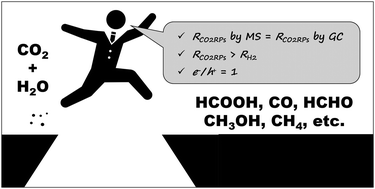
Artificial photosynthesis has recently drawn an increasing amount of attention due to the fact that it allows for direct solar-to-chemical energy conversion. However, one of the basic steps of this process, namely the reduction of CO2 by H2O to afford O2 and CO2 reduction products (CO2RPs) such as HCOOH, CO, HCHO, CH3OH, and CH4, is very difficult to achieve. In contrast to the CO2 reduction in plants and homogenous systems, the reduction of CO2 to CO2RPs over heterogeneous photocatalysts was challenged by the competing reduction of H+ to H2. Unfortunately, most of the research performed so far has focused only on the reduction of CO2, rather than the characterization of the H2O oxidation and H2 production. Moreover, the fact that the heterogeneous photocatalytic reduction of CO2 into CO2RPs by H2O should satisfy several selectivity criteria has often been ignored. Herein, we propose three such evaluation criteria, namely (1) the origin of carbon in CO2RPs (determined using isotopically labeled CO2 (13CO2)), (2) the relative amount of H2 and CO2RPs produced, and (3) the amount of O2 produced by the oxidation of H2O. If all these criteria are satisfied, i.e., the carbons of CO2RPs originate from CO2, the amount of H2 produced is negligible, and a stoichiometric amount of O2 is produced by the oxidation of H2O, then CO2 introduced into the gas phase is believed to be reduced by H2O to CO2RPs in the aqueous phase.
中文翻译:

H 2 O在非均相光催化剂上 成功三相光催化还原CO 2的充要条件
人工光合作用近来引起了越来越多的关注,因为它可以直接将太阳能转化为化学能。但是,此过程的基本步骤之一是通过H 2 O还原CO 2以提供O 2和CO 2还原产物(CO 2 RPs),例如HCOOH,CO,HCHO,CH 3 OH和CH 4,很难实现。与此相反的CO 2减少在植物和同质系统,CO的还原2至CO 2的RP在异构光催化剂是由H的竞争减少挑战+至H 2。不幸的是,到目前为止进行的大多数研究仅集中在减少CO 2上,而不是H 2 O氧化和H 2产生的表征。而且,经常被忽略的事实是,H 2 O将CO 2异质光催化还原为CO 2 RPs应满足几个选择性标准。在这里,我们提出了三个这样的评估标准,即(1)CO 2 RPs中的碳起源(使用同位素标记的CO 2(13 CO 2)确定),(2)H 2和CO 2的相对量产生的RP,以及(3)H 2 O氧化产生的O 2的量。如果满足所有这些条件,即CO 2 RP的碳源自CO 2,则产生的H 2的量可以忽略不计,和的O-化学计量量2为H的氧化所产生的2 O,然后CO 2引入所述气相被认为由H被减小2 O操作CO 2的RP在水相中。
更新日期:2018-02-28
中文翻译:

H 2 O在非均相光催化剂上 成功三相光催化还原CO 2的充要条件
人工光合作用近来引起了越来越多的关注,因为它可以直接将太阳能转化为化学能。但是,此过程的基本步骤之一是通过H 2 O还原CO 2以提供O 2和CO 2还原产物(CO 2 RPs),例如HCOOH,CO,HCHO,CH 3 OH和CH 4,很难实现。与此相反的CO 2减少在植物和同质系统,CO的还原2至CO 2的RP在异构光催化剂是由H的竞争减少挑战+至H 2。不幸的是,到目前为止进行的大多数研究仅集中在减少CO 2上,而不是H 2 O氧化和H 2产生的表征。而且,经常被忽略的事实是,H 2 O将CO 2异质光催化还原为CO 2 RPs应满足几个选择性标准。在这里,我们提出了三个这样的评估标准,即(1)CO 2 RPs中的碳起源(使用同位素标记的CO 2(13 CO 2)确定),(2)H 2和CO 2的相对量产生的RP,以及(3)H 2 O氧化产生的O 2的量。如果满足所有这些条件,即CO 2 RP的碳源自CO 2,则产生的H 2的量可以忽略不计,和的O-化学计量量2为H的氧化所产生的2 O,然后CO 2引入所述气相被认为由H被减小2 O操作CO 2的RP在水相中。









































 京公网安备 11010802027423号
京公网安备 11010802027423号