Biomaterials ( IF 12.8 ) Pub Date : 2018-02-28 , DOI: 10.1016/j.biomaterials.2018.02.048 Francisco Pelaez 1 , Navid Manuchehrabadi 2 , Priyatanu Roy 2 , Harishankar Natesan 2 , Yiru Wang 2 , Emilian Racila 3 , Heather Fong 1 , Kevin Zeng 1 , Abby M Silbaugh 1 , John C Bischof 4 , Samira M Azarin 1
|
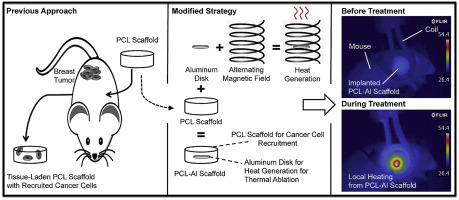
Currently, there are very few therapeutic options for treatment of metastatic disease, as it often remains undetected until the burden of disease is too high. Microporous poly(ε-caprolactone) biomaterials have been shown to attract metastasizing breast cancer cells in vivo early in tumor progression. In order to enhance the therapeutic potential of these scaffolds, they were modified such that infiltrating cells could be eliminated with non-invasive focal hyperthermia. Metal disks were incorporated into poly(ε-caprolactone) scaffolds to generate heat through electromagnetic induction by an oscillating magnetic field within a radiofrequency coil. Heat generation was modulated by varying the size of the metal disk, the strength of the magnetic field (at a fixed frequency), or the type of metal. When implanted subcutaneously in mice, the modified scaffolds were biocompatible and became properly integrated with the host tissue. Optimal parameters for in vivo heating were identified through a combination of computational modeling and ex vivo characterization to both predict and verify heat transfer dynamics and cell death kinetics during inductive heating. In vivo inductive heating of implanted, tissue-laden composite scaffolds led to tissue necrosis as seen by histological analysis. The ability to thermally ablate captured cells non-invasively using biomaterial scaffolds has the potential to extend the application of focal thermal therapies to disseminated cancers.
中文翻译:

用于非侵入性局灶热疗的生物材料支架作为消融转移癌细胞的潜在工具
目前,治疗转移性疾病的治疗选择很少,因为在疾病负担过高之前,转移性疾病往往未被发现。微孔聚(ε-己内酯)生物材料已被证明可以在肿瘤进展早期吸引体内转移的乳腺癌细胞。为了增强这些支架的治疗潜力,对它们进行了修改,以便可以通过非侵入性局灶热疗来消除浸润细胞。将金属盘纳入聚(ε-己内酯)支架中,通过射频线圈内的振荡磁场的电磁感应产生热量。通过改变金属盘的尺寸、磁场强度(固定频率)或金属类型来调节热量的产生。当植入小鼠皮下时,改良后的支架具有生物相容性,并与宿主组织正确整合。通过计算模型和离体表征的结合,确定了体内加热的最佳参数,以预测和验证感应加热过程中的传热动力学和细胞死亡动力学。组织学分析表明,植入的、负载组织的复合支架的体内感应加热导致组织坏死。使用生物材料支架非侵入性地热消融捕获的细胞的能力有可能将局部热疗法的应用扩展到播散性癌症。











































 京公网安备 11010802027423号
京公网安备 11010802027423号